Emulsifiers 101: Who says oil and water don’t mix?
Jennifer Ineman & Joe Schultz | TLT Webinars September 2013
How balancing cooling and lubricity can improve the efficiency of your machinery.
(All figures courtesy of The Lubrizol Corp.)
KEY CONCEPTS
•
Typical common emulsions found in homes include milk, mayonnaise and hair-conditioner products.
•
Several factors can affected performance characteristics of an emulsion.
•
The hydrophilic-lipophilic balance (HLB) measurement is a critical tool for choosing the appropriate emulsifier for a given oil.
WEBINARS: A SERIES FROM TLT
This article is the sixth in a year-long series based on Webinars originally presented by STLE University. In some cases, the Webinar presenter(s) will author the article, like this one, and in others, the Webinar is adapted by a TLT writer.
Jennifer Ineman is a product manager for The Lubrizol Corp. in Wickliffe, Ohio, where she is responsible for providing technical support to metalworking technical consultants and formulators in the field. While at Lubrizol, she has worked in the areas of chemical synthesis and as a formulator in metalworking R&D and has 10 years of experience as a chemist in the lubricant industry.
Joe Schultz is a technical services chemist for Lubrizol’s industrial metalworking additives group, where he provides formulation, application and troubleshooting support for customers and end-users and has worked in the lubricant industry for 18 years. Prior to joining Lubrizol, he worked for a variety of MWF suppliers in roles supporting and formulating specialty metalworking chemistries for producing and finishing steel. You can reach them both at
jine@lubrizol.com and
joe.schultz@lubrizol.com.
STLE University has sponsored dozens of Webinars and podcasts on a wide range of technical topics. To see Jennifer Ineman and Joe Schultz’s Webinar in its entirety, review all STLE University offerings and view the lineup of future events, log on to
www.stle.org. Webinars are $39 to STLE members and $59 for non-members.
 Jennifer Ineman
Jennifer Ineman
 Joe Schultz
Joe Schultz
EVER SINCE THE EMERGENCE OF METALWORKING PRACTICES, there has been a need for fluids that provide both the exceptional lubricating abilities of oils along with the cooling capabilities of water. An oil/water mixture would provide the best qualities of both, but the challenge is that oil and water do not mix on their own. Emulsifiers are molecules that facilitate the combination of two otherwise immiscible liquids to form an emulsion. Examples of common emulsions you might have in your home are milk, mayonnaise and hair-conditioner products.
Using an emulsified fluid as a lubricating coolant can lead to complicated chemical and physical interactions between materials, both favorable and unfavorable. This creates a need to choose lubricants, emulsifiers and other performance additives that fit specific applications and can be combined to form a stable concentrate and emulsion.
Based on an STLE University Webinar titled “Emulsifiers 101,” this article will help familiarize (or refamiliarize) metalworking formulators with (1.) the basic concepts of what emulsions are, (2.) how they are formed and (3.) the types of water-based fluids utilized in metalworking applications.
COMPARISON OF EXTREMES
Before digging into the world of emulsions, it’s important to know the background of why they are desired for metalworking fluid formulation.
Let’s look at a case where a fluid consists entirely of oil and oil-based performance additives. What we observe is:
•
Exceptional lubrication
•
Poor cooling at high temperatures
•
A concern for flash point (heat generation and possible fire)
•
Absorption of tramp oils that dilute the properties of the various performance components
Nevertheless, oil-based technology remains the solution of choice for certain harsh applications such as gear cutting or broaching where an exceptionally high level of lubrication is needed. These straight-oil formulations are also used for applications where the fluid is utilized not only for machining but also to lubricate other moving parts such as in screw machines.
On the other hand, using a fluid that is entirely water with water-based performance additives can yield the following results:
•
Provides the cooling required by the high speeds generated in today’s equipment (water absorbs at five times more heat than air and 2.5 times more than mineral oil).
•
Is non-flammable.
•
Rejects tramp oil, so it tends to sustain component performance longer.
•
Provides relatively poor lubricity.
•
Can contribute to corrosion issues.
Because both oil and water offer unique properties, it makes sense to combine them into one fluid in order to capitalize on the benefits of both.
BASICS
Emulsification is the ability to mix two otherwise immiscible fluids. In the case of MWFs, we are combining polar molecules (water) and non-polar molecules (oil) to create a stable mixture that exploits the beneficial properties of both materials. From grade-school chemistry, you might remember the concept of like-dissolves-like, meaning polar will dissolve polar and non-polar will dissolve non-polar, but they will not dissolve each other. Therefore, when oil and water are mixed, the oil droplets coalesce and rise to the top of the water phase in complete separation (since most oils are lighter than water). How, then, do we combine oil and water to create stable MWFs? The answer is by using emulsifiers.
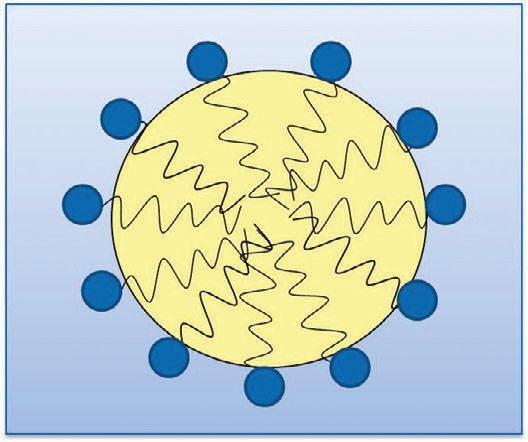 Figure 1. When a surface-active emulsifier is used to combine water and oil, the polar head group (shown in the blue circle) is attracted to water, while the non-polar tail (wavy black line) is attracted to oil, allowing the water and oil to combine.
Figure 1. When a surface-active emulsifier is used to combine water and oil, the polar head group (shown in the blue circle) is attracted to water, while the non-polar tail (wavy black line) is attracted to oil, allowing the water and oil to combine.
An emulsifier is a surface-active chemical that consists of both a polar head group and a non-polar tail. This allows emulsifiers to be attracted to both polar and non-polar molecules. A metalworking emulsion is a composite of water and oil held together by emulsifier molecules. Unfortunately, MWFs tend to be more complex than just simply adding water, oil and emulsifier together.
It is important to point out that when talking about MWFs, we are referring to oil droplets suspended in a continuous water phase. These are known as “oil-in-water” emulsions (
see Figure 2). Water-in-oil emulsions exist, however, but are seldom utilized in metalworking processes and are not covered in this article.
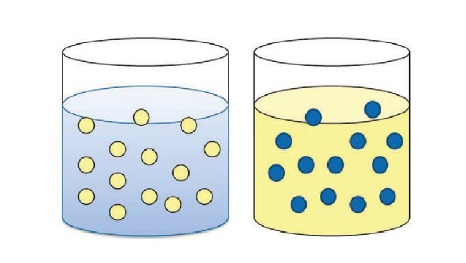 Figure 2. Oil-in-water emulsions are common in metalworking applications (shown on the left); Water-in-oil emulsions typically are not used in metalworking (shown on the right). Oil is represented by yellow; water is represented by blue.
Figure 2. Oil-in-water emulsions are common in metalworking applications (shown on the left); Water-in-oil emulsions typically are not used in metalworking (shown on the right). Oil is represented by yellow; water is represented by blue.
There are many factors that can affect the performance characteristics of an emulsion, including (1.) droplet size, (2.) distribution, (3.) interfacial tension, (4.) hydrophilic-lipophilic balance, (5.) critical micelle concentration, as well as many others. Some of these factors will be covered in this article, while others will be covered in more detail in a follow-up Webinar presentation and in a future TLT article.
TYPES AND PROPERTIES
As discussed earlier, an emulsifier is made up of a polar head group and a non-polar tail. In an oil-in-water emulsion (
see Figure 1), the emulsifier surrounds an oil droplet in such a way that the polar head groups point outward towards the water phase, while the non-polar tail is pointed into the oil droplet. This becomes important when discussing properties of the three different types of emulsifiers—cationic, anionic and non-ionic—and their ability to stabilize an emulsion.
Cationic. A molecule in which the polar head group has a positive charge. These are rarely used in metalworking applications because they typically are not stable in alkaline solutions. MWFs generally require a range of 8.0 to 9.5. Cationic emulsifiers are typically used in low-to-neutral pH industrial cleaning and steel rolling solutions.
Anionic. A molecule in which the polar head group has a negative charge. These are the primary group of emulsifiers utilized in MWFs. Examples include soaps of fatty acids, succinic anhydrides and sulfonates. Anionic emulsifiers provide stabilization of emulsions through electrostatic properties. Each emulsifier aligns itself so that the negatively charged polar head group surrounds each droplet. As these oil droplets flow throughout the emulsion, they inevitably come into contact with one another. The negative charges that surround each droplet will repel each other—similar to the way two negative ends of magnets push each other away (
see Figure 3). By keeping the oil droplets from coming together, this prevents them from coalescing and separating out of solution.
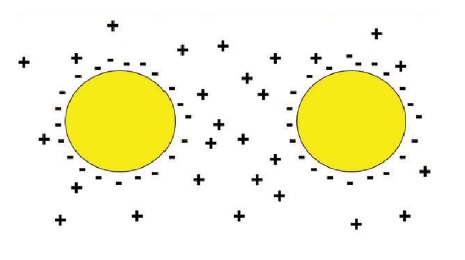 Figure 3. Electrostatic stabilization of anionic emulsifiers: negative charges on the outside of oil droplets repel each other.
Non-ionic.
Figure 3. Electrostatic stabilization of anionic emulsifiers: negative charges on the outside of oil droplets repel each other.
Non-ionic. A molecule whose polar head group is uncharged. These emulsifiers have a place in MWFs as coemulsifiers in which they work in combination with anionic emulsifiers to provide stable emulsions. Examples include amides, esters and ethoxylates. Because these molecules do not have a charge, they provide stability by steric hindrance. The polar head groups tend to be quite large and bulky and point away from each oil droplet. As the droplets encounter one another, the polar head groups will tangle and prevent the droplets from coming together (
see Figure 4).
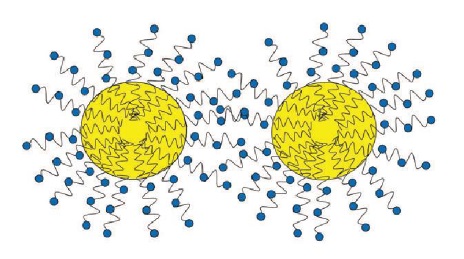 Figure 4. Steric hindrance stabilization of non-ionic emulsifiers: large polar groups hold oil droplets apart from each other.
Figure 4. Steric hindrance stabilization of non-ionic emulsifiers: large polar groups hold oil droplets apart from each other.
Generally emulsions at rest tend to separate over time. This manifests itself first as creaming, then eventually as breaking, where the oil droplets have come together and risen to the top of the water phase (
see Figure 5). This may happen quickly or slowly, depending on the combination of emulsifiers and the additional components present in the formulation. Smaller droplet sizes tend to aggregate more slowly and, therefore, are typically more stable. There are many additional factors that can affect emulsion stability. The type of water that is used to prepare the emulsion, the operation and metal being machined as well as contamination, are just a few examples.
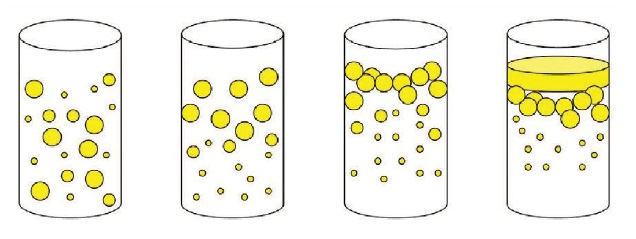 Figure 5. Emulsions offer different stability characteristics and applications demand varying levels of emulsification stability. From left to right: a stable emulsion; the emulsion has begun to separate; the emulsion is creaming (thick white layer on top of the mixture); the emulsion has broken (noticeable oil layer on top of the water phase).
Figure 5. Emulsions offer different stability characteristics and applications demand varying levels of emulsification stability. From left to right: a stable emulsion; the emulsion has begun to separate; the emulsion is creaming (thick white layer on top of the mixture); the emulsion has broken (noticeable oil layer on top of the water phase).
Although emulsion stability is an important characteristic of a MWF, how quickly an emulsion is formed is also key, particularly to the end-user preparing the metalworking emulsion on the machine shop floor. Interfacial tension is the surface tension between two immiscible liquids; in other words, it is the force that keeps the liquids from mixing. An emulsifier lowers the interfacial tension, allowing these non-miscible liquids to intermingle and enabling faster oil droplet formation.
Also keep in mind that it is important for an emulsifier molecule, even though it has both a polar and non-polar part, to be able to move through the oil in which it resides prior to mixing with water to form the emulsion. Combining a low interfacial tension between the liquids and good oil solubility provides turbulence in the mixture, which, in turn, produces concentration gradients. As we know from simple diffusion, molecules want to move from high concentration to low concentration. This movement of molecules creates a phenomenon called necking or droplet formation (
see Figure 6). The more spontaneously an emulsion forms, the less work the end-user must put into preparing their MWF.
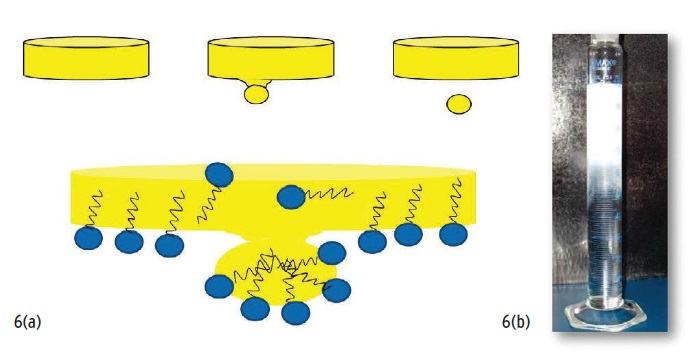 Figure 6. The separation of droplets called necking drives the milky white spontaneous emulsification called bloom (as seen in Figure 6(b)). Fluids characterized by bloom are more likely to provide trouble-free mixing.
Figure 6. The separation of droplets called necking drives the milky white spontaneous emulsification called bloom (as seen in Figure 6(b)). Fluids characterized by bloom are more likely to provide trouble-free mixing.
Up to this point we have talked specifically about emulsifier molecules and how they might form an emulsion. It is important to note that there are many different emulsifier molecules and many different base oils to choose from when formulating a MWF. How does a new chemist to the industry know which emulsifier to choose for a given oil? Furthermore, how do you combine emulsifiers for each unique blend of desired characteristics? There is a powerful tool that can be utilized called the hydrophilic-lipophilic balance (HLB) measurement.
Ideally, an emulsifier exhibits an equal, balanced attraction to water and to the specific oils used. If disproportionately attracted to water (more hydrophilic), the emulsifier molecule is drawn into the water phase and loses contact with the oil. In contrast, if the molecule is too strongly attracted to the oil (lipophilic), the opposite occurs. When the HLB of the emulsifier is fine-tuned to that required by the oil, then the emulsifier will want to go to and remain at the water-oil interface (
see Figure 7). This promotes emulsion spontaneity, as well as stability.
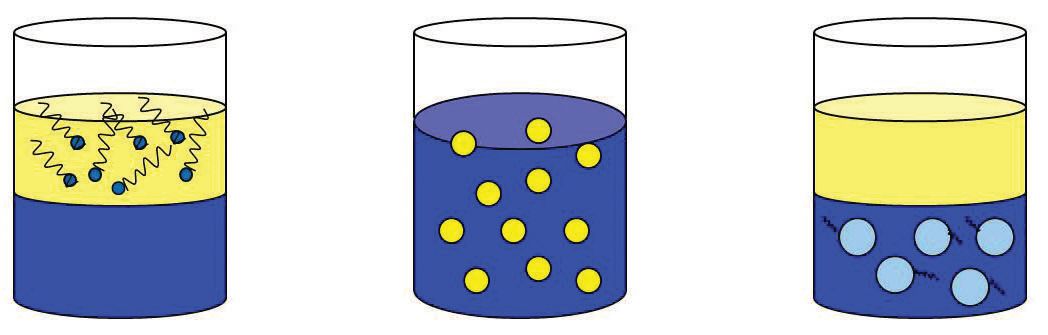 Figure 7. Balancing the HLB of the emulsifiers with the HLB of the oil creates a spontaneous and stable emulsion. From left to right: the emulsifier has a low HLB (stays in the water phase; the emulsifier has an HLB that is fine-tuned to the oil creating an emulsion (water and oil mixed); the emulsifier has a high HLB (stays in the oil phase).
Figure 7. Balancing the HLB of the emulsifiers with the HLB of the oil creates a spontaneous and stable emulsion. From left to right: the emulsifier has a low HLB (stays in the water phase; the emulsifier has an HLB that is fine-tuned to the oil creating an emulsion (water and oil mixed); the emulsifier has a high HLB (stays in the oil phase).
The HLB scale ranges from 0 to 20. A lower HLB value corresponds to an emulsifier that is more lipophilic, and a higher HLB value corresponds to an emulsifier that is more hydrophilic.
Each finished fluid almost always requires more than one emulsifier to combine its various performance components. Each emulsifier has its own HLB measurement, and each oil has a required HLB in order to emulsify it. Some performance additives also contribute to the overall HLB of the formulation.
As a general rule, the required HLBs of some commonly used oils in MWFs are:
•
Vegetable oil: 7-8
•
Paraffinic oil: 8-10
•
Naphthenic oil: 10-12
The HLB requirements of oil-in-water emulsions utilized for most metalworking applications can range anywhere from 7 to 16. With such a broad range, starting with a simple calculation of HLB numbers for different emulsifier combinations can significantly lower the amount of research time needed to find the ideal mixture for a given oil. More experienced formulators tend to be familiar with traditional emulsifier components and can put together a formulation rather quickly without much calculation. For chemists and formulators just starting out in the metalworking industry, using the HLB measurements to put formulations together can greatly reduce the amount of trial and error.
STABILITY
There are many different test methods that are utilized in the industry to measure whether or not an emulsion is stable. One well-known test is the Institute of Petroleum’s IP263. An emulsion is formed and poured into a cassia flask. The flask is then allowed to sit at elevated temperatures overnight. The amount of cream and/or oil is then quantified. A stable emulsion will show no signs of cream or oil separation when evaluated at the end of the test. On the other hand, an unstable emulsion will show cream and/or oil separation.
Typically, different hardness waters are evaluated because they can affect the emulsion stability as well. The ions that reside in hard water interfere specifically with anionic emulsifiers. This is one reason we see combinations of both anionic and non-ionic emulsifiers in MWFs. The two methods of stabilization provided by the different emulsifiers (electrostatic stabilization of anionic emulsifiers and steric hindrance of non-ionic emulsifiers) will help keep droplets separated from each other in different situations.
SOLUBLE OILS
Soluble oils are capable of serving many metalworking applications and can be performance enhanced with a variety of additives (
see Figure 8).
 Figure 8. Example of soluble oil.
Figure 8. Example of soluble oil.
Advantages of soluble oils are:
•
Ability to provide both cooling from the water phase and lubrication from the oil phase.
•
Performance at low concentrations; typically a soluble oil is diluted 5-20 percent into water.
•
Inherent corrosion protection from slight oil film left on the metal surface.
Disadvantages include:
•
Hard water instability
•
Foaming in soft water
•
Subject to microbial attack
•
Finite sump life
•
Poor tramp oil rejection
SYNTHETICS
Synthetic MWFs are water-soluble lubricants that contain no mineral oil and do not require emulsification (
see Figure 9). They are particularly useful for high-speed operations where cooling is more important than lubrication.
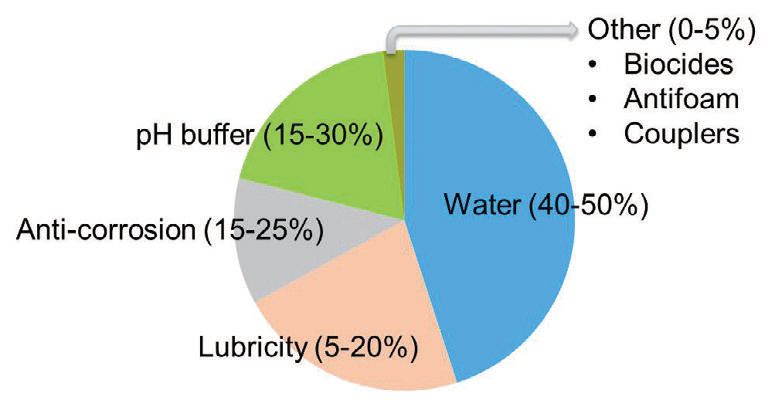 Figure 9. Example of synthetic fluid.
Figure 9. Example of synthetic fluid.
Advantages of synthetics include:
•
pH buffers for bacteria resistance
•
Exhibit good visibility in a clear fluid
•
Are chemically stable
•
Tend to remain clean
•
Are low-foaming
•
Have good wetting properties
•
Provide good settling of swarf (metal fines)
•
Tend to reject tramp oil
Disadvantages include:
•
Tend to have reduced lubricity
•
May develop dry, hard crystalline residue (inherent in the chemistry)
•
Contain pH buffers that can irritate skin due to high alkalinity
•
Have high detergency that can peel paint
•
Are harder to waste-treat because everything is water-soluble
SEMISYNTHETICS
Semisynthetics are the most complex and technically challenging MWFs. They contain both oil-soluble and watersoluble components and, therefore, the concentrates themselves are emulsions (
see Figure 10). They encompass a broad range of product qualities to serve a variety of cost and performance expectations.
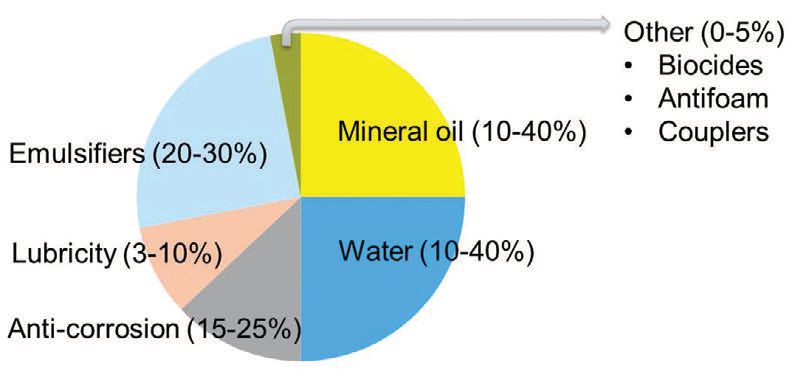 Figure 10. Example of semisynthetic fluid.
Figure 10. Example of semisynthetic fluid.
Advantages of semisynthetics include:
•
Good lubricity
•
Better residue characteristics (not crystalline, not oil)
•
Excellent wetting ability due to a large emulsifier content
•
Easier to waste-treat than synthetic fluids
On the other hand, these products tend to have poor tramp rejection. The high-emulsifier content can readily emulsify tramp oils, which can change and deplete the performance characteristics of the original MWF.
CONCLUSION
The challenge is fine-tuning the combination of emulsifiers in order to obtain the desired performance in MWFs. The goal is to (1.) balance the need for lubricity, (2.) heat dissipation, (3.) corrosion protection, (4.) detergency and (5.) process stability—ultimately, producing a clean operation that can sustain high performance.
The proper balance of cooling and lubricity can improve the efficiency of a metalworking application. Selection of emulsifier chemistry and ancillary additives will impact the service life of the fluid, the protection of the machine and the machined parts, as well as the health and safety of the workers. Proper care of the fluid in-use and education of plant personnel can contribute to long-term savings and worker health and safety.