Surfactant thickening through flow-induced structure formation
Dr. Neil Canter, Contributing Editor | TLT Tech Beat August 2013
A more stable, gel-like material is prepared by passing a surfactant solution through a microfluidic device.
KEY CONCEPTS
•
Surfactants can form long and flexible worm-like micelles under the right conditions of concentration, pH, external additives and temperature.
•
Passing a surfactant solution containing worm-like micelles through a microfluidic device produces stable flow-induced structural phases with viscosity that can increase 1,000 times.
•
The possibility exists to use this technique to thicken water-based lubricants.
SURFACTANTS ARE VERY IMPORTANT COMPONENTS in water-based metalworking fluids because they stabilize complex formulations containing oil-and water-soluble additives. This feature, emulsification, is performed because surfactants contain both hydrophilic and hydrophobic groups, enabling them to interact with both polar materials that are soluble in water and nonpolar materials that are compatible with mineral oil.
A previous TLT article examined the development of unique biosurfactants known as rhamnolipids that are derived from the natural sugar rhamnose (
1). These biosurfactants are water soluble and were found to display good corrosion though they tended to foam, particularly at a pH range of approximately 9 where MWFs are used.
One unique feature of surfactants is their ability in aqueous solutions to congregate into specific nanometer structures known as micelles. Amy Shen, associate professor of mechanical engineering at the University of Washington in Seattle, says, “The most common type of micelle formed by surfactants is spherical at low surfactant concentrations. Surfactants can also form cylindrical micelles.”
Micelle formation is dependent upon a number of factors, including concentration, pH of the solution, external additives and temperature. Under the right conditions, cylindrical micelles can form long and flexible worm-like micelles in the presence of inorganic or organic salts. Apparently, the ionic charge present in the salts acts to repulse the polar surfactant groups, leading to the formation of these more complex micellar structures.
While under flow and shear (the flow has both shear and extension, the extension is important), worm-like micelles can be converted into gel-like species known as flow-induced structural phases (FISPs). Shen says, “FISPs are more complicated structures that are induced by high shear and extension. Under pure shear, the gel-like materials have been known since the 1990s and were called shear induced structures (SIS). SIS are formed at a critical shear rate that enables the worm-like micelles to develop entangled structures that lead to gelling. Unfortunately, this effect is only temporary as once the shear is stopped, the SIS will relax and revert back to a liquid form.”
If a procedure could be found to develop a more stable gel-like structure, then the prospect for more easily increasing the viscosity of aqueous solutions could be realized. This thickening effect may be useful in the performance of water-based lubricants in specific applications.
MICROFLUIDIC DEVICE
Shen and her fellow researchers developed a process for producing a more stable gel-like material by passing a surfactant solution through a microfluidic device containing a series of microposts. The process is shown in Figure 3.
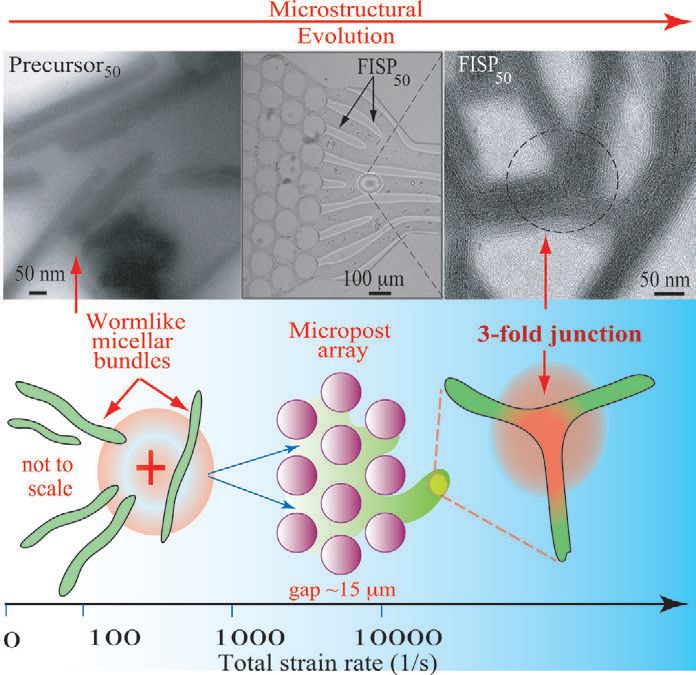 Figure 3. Under conditions that combine shearing and extending, worm-like micelles can be passed through a microfluidic device to prepare stable, flow-induced structural phases that can result in a significant increase in viscosity. (Courtesy of the University of Washington)
Figure 3. Under conditions that combine shearing and extending, worm-like micelles can be passed through a microfluidic device to prepare stable, flow-induced structural phases that can result in a significant increase in viscosity. (Courtesy of the University of Washington)
Shen says, “We set up the device so that the gap between posts was very small (between 5-15 microns) and the fluid was placed under both very high shear and extension rates. Typically, the normal shear for preparing temporary SIS is 10 inverse seconds. We used a process that combined shearing and extension at 5,000 inverse seconds with extensional flow that stretched worm-like micelles like a rubber band.”
The surfactant and salt used in this process are cetyltrimethylammonium bromide and sodium salicylate, respectively. Shen says, “This cationic surfactant and organic salt were chosen because they have exhibited interesting rheological behavior with tiny salt concentration variation in the past.”
The salt-to-surfactant ratio is very important in determining if the resulting mixture will thicken. Shen says, “We found that a salt-to-surfactant ratio of 0.15 produces a solution with a viscosity comparable to water that displays Newtonian behavior. Adding a small amount of salt to change the ratio to 0.2 dramatically thickens the mixture so that the viscosity increases five times.”
If the salt-to-surfactant ratio is further increased to 0.28, then the solution rapidly thins.
The FISP prepared through the microfluidic device from a dilute salt/surfactant mixture increases the viscosity by 1,000 times. In addition, the FISP also exhibits elastic properties. Shen says, “A FISP prepared through the microfluidic device is extremely stable and can be scooped out of the solution. It will dissolve if heated above a temperature of 50 C.”
A single worm-like micelle exhibits a diameter between four and five nanometers and has a length that can range from 50 nanometers to submicron. The diameter of a FISP is much larger and is typically about 100 nanometers. Determining the length of this species is difficult because the structure contains entanglements, branches and crosslinks. Instead the FISP can be characterized by a mesh size between 70 and 100 nanometers.
The structures of worm-like micelles and FISPs were characterized by electron microscopy, as shown in Figure 3.
The formation of a stable FISP means that a thickened, aqueous solution can be prepared at a low surfactant concentration. For application in MWFs, cationic surfactants are not used because they are not compatible with most other additives used.
In more recent work, Shen reports that stable FISPs can be formed by using a mixture of nonionic surfactants. One is polyoxyethylene (20) sorbitan monooleate, while the other is glyceryl laurate. This means that there is now the possibility of developing viscous aqueous solutions with nonionic surfactants that are more commonly used in MWFs.
Additional information on this work can be found in a recent article (
2) or by contacting Shen at
amyshen@uw.edu.
REFERENCES
Canter, N. (2004), “Novel Rhamnolipid Biosurfactants,” TLT,
60 (12), pp. 13-15.
Cardiel, J., Dohnalkova, A., Dubash, N., Zhao, Y., Cheung, P. and Shen, A. (2013), “Microstructure and Rheology of a Flow-Induced Structured Phase in Worm-like Micellar Solutions,”
Proceedings of the National Academy of Sciences,
110 (18), pp. E1653-E1660.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.