Overcoming the inability to break down lignin
Dr. Neil Canter, Contributing Editor | TLT Tech Beat June 2013
New research might led to more effectively utilizing lignin as a biomass raw material.
KEY CONCEPTS
•
Some microorganisms such as
Streptomyces bacteria are able to degrade the highly stable biopolymer, lignin in a tightly regulated manner providing an opportunity to better understand the metabolic pathway used.
•
A key intermediate is the phenolic derivative, protocatechuate (PCA), which can readily be converted into useful compounds such as biofuels.
•
New research has determined how the transcription factor known as PcaV regulates the genes in
Streptomyces bacteria used to activate the PCA pathway.
LIGNIN IS AN AROMATIC-BASED BIOPOLYMER that can constitute as much as a third of plant biomass. As research continues to utilize biomass as an alternative feedstock to petroleum, the key is to work with raw materials derived from non-edible, biobased sources so as not to interfere with food supply.
Lignin utilization represents a potentially good approach because it is readily available and cannot be used as a food. But lignin is a high stable polymer that is very difficult to break down in nature. This is for good reason because lignin is the main structural building block for plants.
From a lubricant standpoint, lignin contains some useful chemical functionality such as phenols, alkoxy and hydroxyl groups. In a previous TLT article, lignin-based additives were developed that show promise as extreme pressure additives in grease applications (
1). The presence of the phenol groups also gives the lignin-based additives some antioxidant characteristics.
Jason Sello, professor of chemistry at Brown University in Providence, R.I., discusses the challenges faced with breaking down lignin. He says, “While there are many microorganisms that cannot break down lignin, some bacteria and fungus can depolymerize this material. The pathway used converts polymeric lignin to smaller aromatic building blocks that are ultimately transformed into a common phenol derivative called protocatechuate (PCA).”
PCA is a very important compound because it can be converted by microorganisms into beta-ketoadipate, which is then broken down into the readily usable acetyl-coenzyme and succinyl-coenzyme A. Sello says, “Both of these products can be readily converted into either triglycerides, which are the precursors of biofuels such as biodiesel, or high-value chemicals such as polyketide antibiotics.”
One of the key aspects to trying to work with lignin is to figure out how the metabolic pathways through which microorganisms degrade lignin are regulated. Knowledge of the regulation can be critical for engineering microorganisms to convert lignin into biofuels or chemicals. One group of microorganisms in which lignin degradation is tightly regulated is
Streptomyces bacteria.
“In early experiments, we found that
Streptomyces bacteria turned on the expression of genes necessary for the consumption of PCA when they were exposed to the chemical. It is known that there is a large group of proteins called transcription factors that can control the expression of genes and, as a consequence, affect the ability of a bacterium to undertake certain pathways,” Sello says. “We discovered that the transcription factor, PcaV is present in
Streptomyces and is involved in regulating the genes that encode enzymes involved in the conversion of PCA into beta-ketoadipate and ultimately succinyl CoA and acetyl CoA. We predicted that PcaV enabled the bacteria to detect PCA and to regulate the expression of the genes for PCA metabolism.”
If it can be determined how PcaV regulates the genes that enable
Streptomyces bacteria to use PCA, then a process can be developed to more fully utilize lignin as a biomass raw material. Research has now been conducted that shows how PcaV is able to regulate the PCA pathway.
PCAV-PCA COMPLEX
Sello, in collaboration with Rebecca Page, biology professor in the department of molecular biology, cell biology and biochemistry at Brown University, determined how PcaV functions to activate the PCA pathway, which is essential for the polymerization of lignin derived compounds. The researchers found through the use of electrophoretic mobility shift assays (EMSAs) that PcaV binds to the sections of the DNA, controlling the PCA pathway forming a complex.
Sello says, “We determined that this complex is formed by first running DNA on an agarose gel in the presence and absence of PcaV. In the presence of the protein, the rate at which DNA migrates is very slow, which is indicative of the formation of a complex between PcaV and DNA. Upon addition of PCA to the PcaVDNA complex, the DNA migrates through the gel at the same rate that it does in the absence of PcaV. This observation indicates that PcaV cannot bind DNA in the presence of PCA.”
The effect of this complex is to repress expression of the PCA degrading genes, shutting off the pathway. Upon the addition of PCA, the PcaV-DNA complex falls apart and the expression of the genes become activated.
To gain insight into how PCA influences the capacity of PcaV to bind DNA, the researchers used a technique called protein crystallography to generate crystals of the PcaV-PCA complex. An image of this complex is shown in Figure 2.
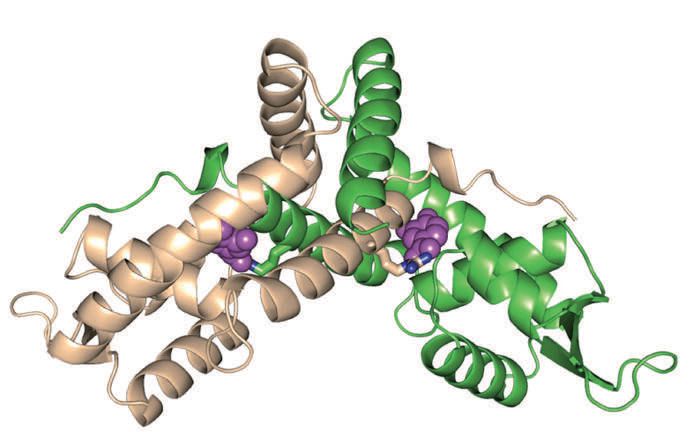 Figure 2. The complex formed between the transcription factor, PcaV and a phenolic derivative, protocatechuate (PCA) is pivotal in regulating a pathway used by Streptomyces bacteria to degrade lignin. (Courtesy of the Sello lab/Brown University)
Figure 2. The complex formed between the transcription factor, PcaV and a phenolic derivative, protocatechuate (PCA) is pivotal in regulating a pathway used by Streptomyces bacteria to degrade lignin. (Courtesy of the Sello lab/Brown University)
Sello says, “We found through analysis of the crystal structure that the arginine- 15, an amino acid in PcaV is one of several that interact with PCA. The interaction is an especially strong hydrogen bond between the amino acid and the PCA. Given the apparent strength of this bond, we suspect that it is the strongest contributor to the interaction between PcaV and PCA.”
Mutations of the PcaV gene were introduced to determine the extent to which arginine-15 was important for PCA binding. Sello says, “We first prepared a version of PcaV with alanine in place of the arginine. We were intrigued to find that protein did not bind with either PCA or with DNA. A second variation involved replacing the arginine with lysine, an amino acid that also has a positive charge, but a different structure. This protein bound DNA, but could not bind PCA. These observations show that arginine-15 is critical for both the binding of PcaV and PCA.”
Since arginine-15 is critical for DNA and PCA binding, Sello hypothesizes that PcaV adopts two different conformations. In one conformation, arginine-15 is engaged in interactions that favor DNA binding and in the other it binds PCA and cannot interact with other amino acids in a way that enables DNA binding. It is apparent that arginine-15 is a gatekeeper or switch that dictates the conformation for PcaV. Unfortunately, the researchers could not produce crystals of a PcaV-DNA complex to understand the interactions that the arginine makes when PcaV binds DNA in the absence of PCA.
Future work will focus on proving that the carbon from aromatic derivatives of lignin can be processed by microorganisms into biofuels and high valued compounds such as antibiotics. Sello says, “We plan to feed isotopically labeled lignin derivatives to microorganisms and determine if the isotope labeled carbons can end up in antibiotics and triglycerides.”
Additional information about this research can be found in a recent article (
2) or by contacting Sello at
jason_sello@brown.edu.
REFERENCES
1.
Canter, N. (2011), “Environmentally Friendly Extreme Pressure Additive,” TLT,
67 (10), pp. 10-11.
2.
Davis, J., Brown, B., Page, R. and Sello, J. (2013), “Study of PcaV from
Streptomyces coelicolor Yields New Insights into Ligand-Responsive MarR Family Transcription Factors,”
Nucleic Acids Research,
41 (6), pp. 3888-3900.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.