Low-temperature production of crystalline silicon
Dr. Neil Canter, Contributing Editor | TLT Tech Beat April 2013
A more efficient, low-temperature process uses a liquid-metal electrode for preparing crystalline silicon.
KEY CONCEPTS
•
Carbothermal reduction is an expensive and energy-intensive process used for preparing crystalline silicon.
•
A potentially more efficient, low-temperature electrodeposition process has been developed to prepare crystalline silicone.
•
The key to the process is the use of a liquid-metal electrode that acts to reduce the silicon precursor and solubilize the silicone until it reaches a supersaturated state that leads to crystallization.
CRYSTALLINE SILICON IS A VERY IMPORTANT RAW MATERIAL due to its use in semiconductor electronics applications such as computers. Another use for crystalline silicon is in solar cells that capture energy from the sun.
Research has been ongoing to find ways to reduce the cost of solar cells to enable this form of energy to become more competitive. In a previous TLT article, work conducted to develop a solar coating or paint was described (
1). Quantum dots prepared from cadmium sulfide and cadmium selenide were coated on titanium dioxide nanoparticles and applied to a conductive surface to form a solar paint. This more cost-effective approach produced a coating that converted solar energy to electricity and has potential to be commercialized.
One of the major reasons for the high cost is the energy-intensive process known as carbothermal reduction used currently to prepare crystalline silicon. While silicon is readily available as silicon dioxide (or silica) in about 40% of the earth’s crust, the conversion to crystalline silicon is very inefficient. Stephen Maldonado, assistant professor of chemistry and applied physics at The University of Michigan in Ann Arbor, Mich., says, “In a multisequence fashion, carbothermal reduction converts silica initially to raw silicon, then metallurgical grade silicon and finally to crystalline silicon. The process takes place at temperatures well in excess of 1,000 C in an electric furnace. One other problem is that undesirable byproducts such as carbon dioxide are also formed.”
A second approach used to prepare crystalline silicon is electrodeposition that involves reducing an oxidized form of the desired element onto an electrode in the presence of an applied voltage. Maldonado says, “Electrodeposition of silicon has been tried at low temperatures and high temperatures. In the former case, the silicon produced is too impure and is amorphous, which means that high-temperature annealing and purification is still required in the same fashion as carbothermal reduction. High-temperature electrodeposition (temperatures > 700 C) can be done, but has not proven efficient enough to supplant carbothermal reduction reactions.”
A need exists for a more efficient process to prepare crystalline silicon at low temperatures. Such a process has now been developed.
LIQUID-METAL ELECTRODE
Maldonado and his fellow researchers have determined that crystalline silicon can be prepared at low temperatures through an electrodeposition process using a liquid-metal electrode instead of a traditional electrode. He says, “We have been working with liquid- metal electrodes as a means for reducing dissolved species in solution and for recrystallization applications through a process known as electrochemical liquid-liquid-solid. In our current work to produce crystalline silicon, we are working with a liquid gallium electrode.”
The researchers used a two-compartment electrochemical cell to effect the deposition of silicon. Silicon tetrachloride was utilized as the oxidized silicon precursor. The system was pressurized at 400 psi to offset the volatility of silicon tetrachloride, and the gallium electrode was placed in propylene carbonate with 0.2 M tetrabutylammonium chloride.
Experiments were conducted at temperatures ranging from ambient up to 200 C. A deposition current of 20 mA cm
-2 was used.
As shown in Figure 3, electrodeposition of the silicon on the gallium electrode was achieved through reduction of silicon tetrachloride. In this particular case, the silicon present on the gallium electrode in Figure 3 was formed at a temperature of 100 C after two hours.
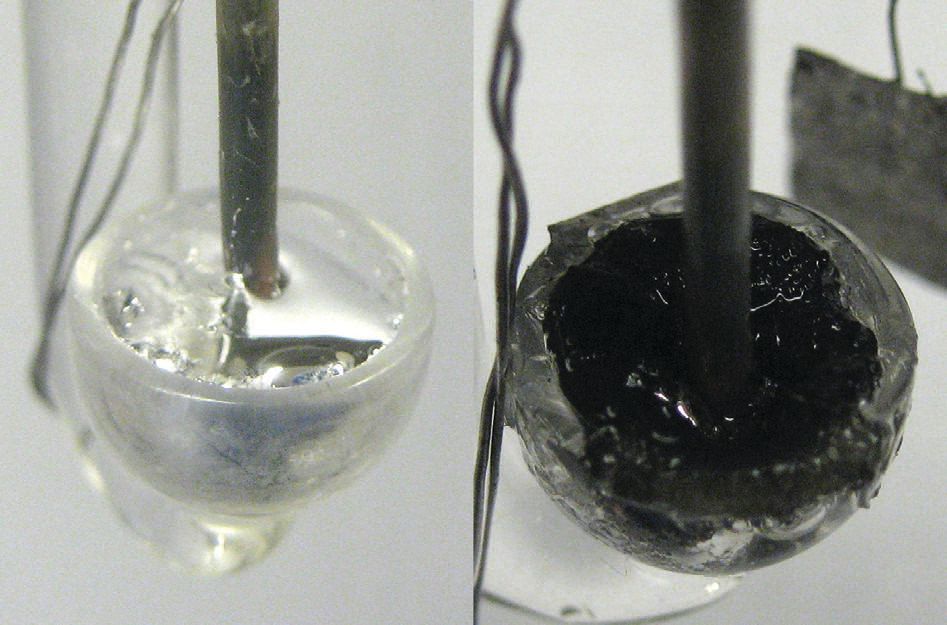 Figure 3. The liquid gallium electrode (shown on the left) is covered with crystalline silicon (shown on the right) through a low-temperature electrodeposition process. (Courtesy of The University of Michigan)
Figure 3. The liquid gallium electrode (shown on the left) is covered with crystalline silicon (shown on the right) through a low-temperature electrodeposition process. (Courtesy of The University of Michigan)
The process takes place in four steps. Initially, gallium reduces silicon tetrachloride to silicon. The newly formed silicon dissolves in gallium until it reaches a supersaturated state when it will spontaneously form nuclei that come out of solution in the last step as crystals.
Maldonado says, “In a similar fashion to preparing crystalline rock candy from a super-saturated aqueous solution of sugar, we are using gallium as a crystallization solvent to expedite the crystallization of silicon.”
The lowest temperature where crystalline silicon was obtained was 80 C. Maldonado adds, “This result is significantly better than the previous low temperature by at least 500 degrees.”
Several factors were studied to determine how to optimize the process. Maldonado says, “We found that increasing the temperature leads to bigger crystals. But moving to high pressure did not lead to better crystallization of silicon. The biggest factor of all is the type of liquid metal used to solubilize the silicon. Besides gallium, a second common liquid metal that can be evaluated is mercury. But there are lots of other options that we intent to study in this wide open field.”
Future work will also evaluate the use of another silicon source because silicon tetrachloride is too susceptible to hydrolysis. Maldonado says, “We believe that 80 C is not the lowest temperature that can be used to generate crystalline silicon. Ultimately, our goal is to find reaction conditions for preparing crystalline silicon at room temperature.
Further information can be found in a recent article (
2) or by contacting Maldonado at
smald@umich.edu.
REFERENCES
1.
Canter, N. (2012), “Solar Paint,” TLT,
68 (4), pp. 12-13.
2.
Gu, J., Fahrenkrug, E. and Maldonado, S. (2012), “Direct Electrodeposition of Crystalline Silicon at Low Temperatures,”
Journal of the American Chemical Society,
135 (5), pp. 1684-1687.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.