Factor-limiting battery life
Dr. Neil Canter, Contributing Editor | TLT Tech Beat March 2013
Researchers found that lithium ions migrated into the copper current collector used in the anode.
KEY CONCEPTS
•
Evaluating the aging characteristics of lithium-ion batteries under real-life cycling driving cycles has not been done.
•
A new study on a commercially available lithium-ion battery shows that lithium ions migrated to the copper current collector used in the anode.
•
The presence of lithium ions in the anode’s copper current collector may negatively affect the battery’s lifetime performance.
WHILE THERE’S BEEN GREAT INTEREST IN USING BATTERIES IN AUTOMOBILES in order to help increase the Corporate Average Fuel Economy, negative performance issues have to be overcome. Operating problems have been encountered with the most widely used type, lithium-ion batteries, which have been found to catch fire under extreme conditions.
A second concern is also present about the availability of lithium as a raw material to meet increased demand as the use of lithium-ion batteries in the marketplace increases.
These factors have led researchers to look at alternative materials in developing batteries. For example, a recent TLT article discussed the development of titanium dioxide as an alternative anode material to carbon and the potential use of a sodium-ion battery (
1). Amorphous titanium dioxide nanotubes have been developed that convert into a more crystalline structure during the first several charge, discharge cycles. Crystalline structures are desired because they facilitate the intercalation of lithium ions into electrode materials.
One other factor that has not been taken into consideration is how effectively batteries perform over a long operating period. STLE-member Bharat Bhushan, Ohio Eminent Scholar and Howard D. Winbigler Professor of Mechanical Engineering at The Ohio State University in Columbus, Ohio, says, “Not many studies have been done to evaluate the aging characteristics of lithium-ion batteries under real-life driving cycles in the past. This issue has now taken on a greater degree of importance because a typical battery used in an automobile costs $10,000, which is considerably more than the batteries we all use in flashlights.”
Initial work done by Bhushan, along with Ohio State colleagues Suresh Babu, professor of materials science and engineering, and Dr. Shrikant Nagpure, postdoctoral researcher, found that lithium ions build up onto the surface of the anode, causing the battery to lose charge capacity. Bhushan says, “During charging, lithium ions migrate from the cathode to the anode, while they move back to the cathode during discharging. It is important for lithium ions to be on the cathode and no place else so they are available for the next battery cycle.”
But the problem with seeing lithium ions on the surface of the anode has led the researchers to determine if this ion is present within components used in the battery.
NEUTRON-DEPTH PROFILING
New work by Bhushan and his fellow researchers has detected the presence of lithium ions in the copper current collector currently used in the anode. The current collector is an important component in a battery because it serves as a conducting path from the electrode to the outside circuit.
The technique used to detect lithium ions is known as neutron-depth profiling. Past attempts to quantify the concentration of lithium ions in batteries have not been successful because the thin beryllium windows in electron-based analytical techniques prevents low-energy x-rays from any element with an atomic number lower than that of carbon from being detected.
Neutron-depth profiling involves the detection of energy particles that are produced from the reaction of lithium ions with a beam of incident neutrons. The two particles produced from this process are
4He and
3H.
The researchers selected a commercially available lithium-ion battery to study. The battery contained a graphite anode, a cathode containing lithium, iron-phosphate nanoparticles (40-50 nanometers in diameters) and a 1:1 electrolyte mixture of ethylene carbonate and dimethyl carbonate that includes lithium hexaflurorphosphate salt. Bhushan says, “We selected this battery type because it is widely used by the automotive industry.”
The battery has an operating voltage of 3.3 V and a nominal discharge capacity of 2.3 Ah. The researchers cycled the battery until it reached approximately 80% of its rated capacity. The battery was then completely discharged and the cell opened under an inert atmosphere to evaluate the components.
The disassembled lithium-ion battery is shown in Figure 2(a). A view of the copper current collector used in the anode is shown in Figure 2(b) between the two graphite strips. In the cathode, aluminum is used as the current collector and Figure 2(c) shows this metal between two strips of the cathode material.
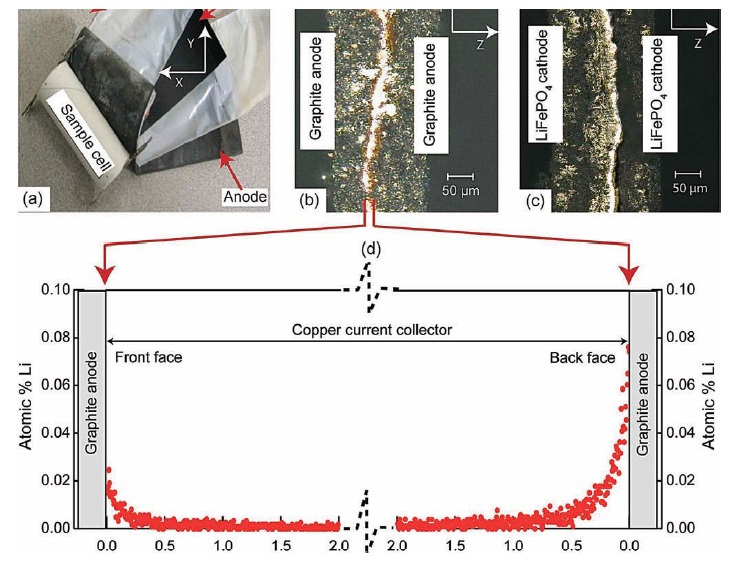 Figure 2. After a commercially available lithium-ion battery underwent an aging study, the detection of lithium in the copper current collector may negatively impact its performance. (Courtesy of The Ohio State University)
Figure 2. After a commercially available lithium-ion battery underwent an aging study, the detection of lithium in the copper current collector may negatively impact its performance. (Courtesy of The Ohio State University)
Neutron-depth profiling of the copper current collector on the anode is shown in Figure 2(d). The researchers evaluated the lithium concentration on the two faces of the current collector. The maximum on the front face is 0.025% atomic lithium, while the maximum on the back face is 0.08% atomic lithium.
This result is a surprise to the researchers. Bhushan says, “Lithium ions are not supposed to migrate into the anode’s current collector, and this result may negatively affect the lifetime performance of the battery.”
The hope is that this work will be used to accelerate optimization of the composite electrode structures that will minimize the movement of lithium ions away from the circuit between the cathode and the anode.
Bhushan says, “Future work will involve using atomic force microscopy to better understand this process at the nanoscale in order to determine a potential mechanism. Further information on this work can be found in a recent reference (
2) or by contacting Bhushan at
bhushan.2@osu.edu.
REFERENCES
1.
Canter, N. (2012), “Titanium Dioxide: New Anode Material for Batteries,” TLT,
68 (2), pp. 12-13.
2.
Nagpure, S., Downing, R., Bhushan, B. and Babu, S. (2012), “Discovery of Lithium in Copper Current Collectors used in Batteries,”
Scripta Materialia,
67 (7-8), pp. 669-672.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.