Improved sulfur removal from petroleum-based fuels
Dr. Neil Canter, Contributing Editor | TLT Tech Beat March 2013
A new sulfur adsorbent containing nanoparticles is highly effective at removing hydrogen sulfide.
KEY CONCEPTS
•
Emission Control Areas in specific regions have been established to reduce sulfur emissions in marine applications.
•
Current technology for removing sulfur from fuel is ineffective because the process used with alkylamines is cumbersome and unable to treat high-temperature fuel steams.
•
A new nanostructured adsorbent containing nanofibers of zinc and titanium dioxide is highly effective at removing hydrogen sulfide.
EMISSIONS REDUCTION IS AN ONGOING TREND IN THE TRANSPORTATION SECTOR. This process is driven by increasingly tighter restrictions, particularly in the developed world.
An important objective for researchers in this field has been to reduce NO
x levels in diesel engines. One of the strategies has been to find more cost-effective catalysts without the use of the precious metals—palladium, platinum and rhodium.
In a previous TLT article, researchers developed an effective NO
x reduction catalyst without using a precious metal (
1). Instead a catalyst based on the silicate mineral mullite was prepared using a combination of four metal oxides (manganese, cerium, samarium and strontium). Testing showed that 45% performance was achieved as compared to platinum. The key to the performance is the presence of a manganese dimer on the surface of the catalyst.
Reduction of sulfur emissions is also becoming an important issue. While much of the developed world has moved to ultra-low, sulfur diesel for use in automobiles and trucks, high-sulfur fuel is still used in oceangoing vessels.
Currently, the maximum sulfur content for a ship’s bunker fuel is 3.5%. But Emission Control Areas (ECAs) have been established in Europe (Baltic and North Seas), and a new ECA was just set up within 200 miles of the North American continent in August 2012. In the case of the North American regulation, ships will be required to use fuels that have a maximum sulfur level of 1%. By 2015 the limit will drop to 0.1%.
This trend means there is an opportunity to look for technologies that can more efficiently remove sulfur from fuel. Prashant Jain, assistant professor of chemistry at the University of Illinois in Urbana, Ill., says, “The current technologies used to remove the organosulfur in the form of hydrogen sulfide from petroleum-based fuels have been alkylamines. The problem in using them is that such a liquidphase extraction is very cumbersome. In addition, high-temperature fuel streams (above 400 C) have to be cooled for the alkylamine treatment step, which comes at an energy cost.”
This temperature issue led researchers to start working with metal oxides such as zinc oxide, which have stability at high temperatures. These oxides are more effective but still are not as efficient because only the outermost layers adsorb sulfur, leading to limited uptake and thermal disintegration.
Development of a more effective sulfur adsorbent containing nanoparticles appears to be the most logical way to significantly improve performance because it will maximize surface area. Such a nanostructured adsorbent has now been developed.
METAL OXIDE NANOFIBERS
Jain led a research team, in collaboration with Mark Shannon, professor of mechanical science and engineering at the University of Illinois, which has developed a new sulfur adsorbent containing nanofibers of zinc and titanium oxide. This material is highly effective at adsorbing hydrogen sulfide due to its nanostructured morphology.
As for the composition of the fibers, Jain says, “Zinc oxide reacts with hydrogen sulfide but is vulnerable to reduction because many fuel streams contain hydrogen. This reduction process converts zinc oxide to zinc, which is ineffective as an adsorbent. We turned to using titanium dioxide because this species is much more resistant to reduction.”
The researchers prepared the zinc titanates with zinc to titanium ratios of 3.7:1 and 1:1, respectively. The former is known as ZT1, while the latter is designated as ZT2. A procedure known as electrospinning was used to prepare the nanofibers. Jain says, “Electrospinning has been a very popular process for the last 15 years. In the synthesis of ZT2, a sol-gel colloid containing the raw materials and a 10% solution of polyvinylpyrrolidone was prepared. This mixture was sprayed onto an electrically charged plate maintained at a high potential of 20 kilovolts.”
The result is the formation of nanoscale fibers. For ZT1, the mean fiber diameters were 435 nanometers +/-165, and the grains constituting the fiber exhibited an average diameter of 40 nanometers +/-14. With ZT2, the mean fiber diameters were 488 nanometers +/- 289, and the grains had an average diameter of 70 nanometers +/- 32.
Initially, the adsorbent candidates were reacted with 4% hydrogen to assess their ability to resist reduction. Due to its higher titanium content, ZT2 displayed better performance and was chosen as the adsorbent to be used in testing with hydrogen sulfide at temperatures ranging from 500 C to 650 C.
Thermogravimetric analysis was used to evaluate the ability of ZT2 to adsorb hydrogen sulfide and then regenerate. Initially, a 1% stream of hydrogen sulfide in nitrogen was used and the change in the weight of the adsorbent measured.
X-ray diffraction analysis shows that the main zinc-sulfide phase generated during the sulfur adsorption process is wurtzite, which is less common and less stable than sphalerite. This is beneficial because the wurtzite more readily oxidizes back to zinc oxide during the regeneration step, run in the presence of a 3% stream of oxygen in nitrogen.
The catalyst showed good durability without any loss in hydrogen sulfide adsorption performance in the six cycles run by the researchers. Jain says, “We could have run more cycles but were restricted by the availability of the facility we used.”
Figure 1 shows a secondary electron image of the nanofibers. Scanning electron microscopy showed that the nanofibers did not appear to disintegrate after a single cycle.
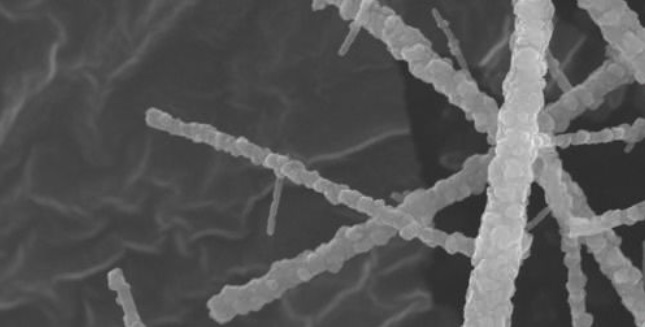 Figure 1. Nanofibers prepared from zinc and titanium dioxide have been found to effectively remove hydrogen sulfide because of their greater surface area. They may potentially be used to remove hydrogen sulfide from petroleum-based fuels. (Courtesy of University of Illinois)
Figure 1. Nanofibers prepared from zinc and titanium dioxide have been found to effectively remove hydrogen sulfide because of their greater surface area. They may potentially be used to remove hydrogen sulfide from petroleum-based fuels. (Courtesy of University of Illinois)
For future work, Jain says, “We are going to address how effective the nanostructured zinc titanate is at adsorbing other sulfur species such as mercaptans and disulfides directly without requiring conversion into hydrogen sulfide. One approach is to prepare a nanostructure based on nickeldoped, zinc oxide because the nickel dopants are quite effective at removing mercaptans.”
Further information can be obtained from a recent article (
2) or by contacting Jain at
jain@illinois.edu.
REFERENCES
1.
Canter, N. (2012), “Effective NO
x Reduction without using Precious Metals,” TLT,
68 (11), pp. 6-7.
2.
Behl, M., Yeom, J., Lineberry, Q., Jain, P. and Shannon, M. (2012), “A Regenerable Oxide-Based H
2S Adsorbent with Nanofibrous Morphology,”
Nature Nanotechnology,
7 (12), pp. 810-815.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.