Systematic identification of new refrigerants
Dr. Neil Canter, Contributing Editor | TLT Tech Beat December 2012
A new study examines refrigerants that exhibit low global-warming potential.
KEY CONCEPTS
•
A systematic approach has been initiated to identify potential refrigerants that exhibit low global-warming potential.
•
An initial list of 56,000 candidates has been reduced through screening to just over 1,200 compounds.
•
Most of the remaining candidates are members of the hydrooflurinated olefin (HFO) series. Researchers intend to reduce the list to about 20 candidates that can be tested experimentally.
THE PURPOSE OF REFRIGERATION is to extract heat from some space such as an air-conditioned room or the interior of a refrigerator. Most systems operate by a process known as the vapor-compression refrigeration cycle. In this fashion, a volatile liquid or refrigerant acts to remove the heat from the space and dissipates it to a “heat sink” (typically outside air) when it moves through the condenser.
The use of a refrigeration lubricant is crucial in ensuring this process is done efficiently. Among its functions are (1.) lubricating internal parts, (2.) removing heat generated during compression, (3.) cleaning the system, (4.) acting as a fluid seal and (5.) reducing energy requirements.
One very important aspect is that the refrigerant must be miscible with the lubricant. Good miscibility is important in order to ensure that the refrigeration lubricant returns to the compressor during operation.
The choice of a refrigerant has been changing over the past 25 years since the ratification of the Montreal Protocol in 1987. At that time, chlorofluorocarbons (CFCs) had been successfully used because of their stability, nonflammability and low toxicity. Unfortunately, that feature also proved to be detrimental as CFCs were found to deplete ozone in the Earth’s stratosphere.
The Montreal Protocol declared that CFC refrigerants should be phased out in developed countries in 1995. This led the industry to move first to hydrochlorofluorocarbons (HCFCs) as an interim measure and then to hydrofluorocarbons (HFCs) such as R134a. But both of these classes of refrigerants contribute to global warming. HCFC-22 has a global-warming potential (GWP) of 800 and HFC-134a has a GWP of 1300.
For a more detailed review of registration lubricants, check out the
feature article in the
December 2009 TLT (available digitally at
www.stle.org) (
1).
An industry-wide search is now in progress for refrigerants with low GWPs. One of the key motivations arises from a European Union directive that dictates that the refrigerant in automotive air-conditioning systems has a GWP no higher than 150.
Hydrofluoroolefins (HFOs), such as R1234yf, have emerged as options, but there is still a need for a more systematic process to find out if there are more suitable candidates. Such a process has now been initiated.
FILTERING OUT POTENTIAL CANDIDATES
Drs. Andrei Kazakov, physicist, Mark McLinden, chemical engineer, and Michael Frenkel, director of the Thermodynamics Research Center at the National Institute of Standards and Technology in Boulder, Colo., are conducting a study to identify potential refrigerants that exhibit low GWP. They settled on a “time horizon” for GWP of 100 years. McLinden says, “This timing is consistent with most regulations.”
Initially, the researchers identified over 56,000 molecules as initial candidates. These substances have 15 or fewer atoms and only contain carbon, hydrogen, oxygen, fluorine, chlorine, bromine, nitrogen and sulfur.
As a result, the researchers decided to evaluate the GWP of these candidates. A simple definition of GWP is provided by McLinden who says, “GWP is the ability of a substance to heat the atmosphere relative to carbon dioxide.”
Values of GWP derived from experimental data are presently available only for a small number of compounds. Frenkel says, “Knowing that there was not much experimental data available, we used estimation methods to determine the GWP for all of the 56,000 compounds screened.”
Two of the factors used to estimate GWP are the radiative efficiency and stability (or lifetime) of a substance. Radiative efficiency measures the ability of a compound to trap radiation coming from earth and, therefore, prevent it from escaping into space.
Frenkel adds, “Radiative efficiency is measured by examining how effectively compounds absorb radiation in the infrared region.”
The second factor refers to how long the substance will survive in the atmosphere before being eliminated through reaction with another compound. Frenkel says, “In the atmosphere, many interactions occur among compounds, leading some to have very short lifetimes. Many compounds can be removed from the atmosphere through chemical reactions with hydroxyl radicals, ozone, the nitrate anion and chlorides, ultraviolet radiation and hydrolysis. We decided to just focus on how the compounds screened reacted with hydroxyl radicals because it is the dominant removal mechanism for the majority of known low-GWP compounds.”
The initial GWP screen only reduced the number of candidates by approximately 4,000. Further parameters were used to filter out candidates. The properties examined included toxicity, flammability, critical temperature and stability.
Most of the candidates were removed by the toxicity and critical temperature screens. Frenkel says, “The critical temperature represents the highest temperature at which a substance can exist as a liquid. This is an important property in the compression refrigeration cycle, where the refrigerant is converted back and forth between the liquid and the gas states. In our study, we looked for compounds with critical temperatures between 300 K and 550 K.”
The reason for using this temperature range is that most refrigerants have critical temperatures between 343 K and 395 K.
After the screening, the researchers reduced the number of candidates to just over 1,200. Figure 1 summarizes the process used. The candidates have been organized into three classes: halogenated hydrocarbons, halogenated oxygenates and halogenated compounds that contain nitrogen or sulfur. It should be noted that the largest class of halogenated hydrocarbons is based on linear olefins that are members of the HFO series. The prevalence of halogenated compounds is due to the flammability filter.
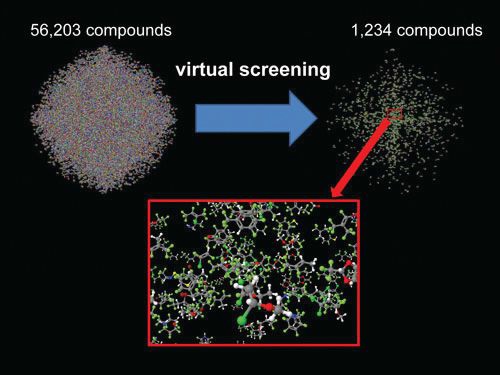 Figure 1. A project has been initiated to identify new refrigerant candidates that combine good performance with GWP values below 150. After starting with over 56,000 compounds, researchers have trimmed the number down to just above 1,200. (Courtesy of Kazakov/NIST)
Figure 1. A project has been initiated to identify new refrigerant candidates that combine good performance with GWP values below 150. After starting with over 56,000 compounds, researchers have trimmed the number down to just above 1,200. (Courtesy of Kazakov/NIST)
This represents only the initial stage of work on this project. Frenkel says, “We are in the process of evaluating the remaining 1,200 candidates to determine how well they will perform as refrigerants. This work is accomplished by examining how well they function under standard refrigeration conditions.”
The researchers hope to end up with about 20 candidates that can then be tested experimentally. Additional information can be found in a recent reference (
2) or by contacting Frenkel at
michael.frenkel@nist.gov.
REFERENCES
1.
Canter, N. (2009), “Refrigeration Lubricants: Transitioning to New Refrigerants,” TLT,
65 (12), pp. 30-39.
2.
Kazakov, A., McLinden, M. and Frenkel, M. (2012), “Computational Design of New Refrigerant Fluids Based On Environmental, Safety and Thermodynamic Characteristics,”
Industrial and Engineering Chemical Research,
51 (38), pp. 12537-12548.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.