Determination of structure of new carbon allotrope
Dr. Neil Canter, Contributing Editor | TLT Tech Beat November 2012
Researchers use experimental data to predict the structure of M-carbon formed at room temperature under pressure.
KEY CONCEPTS
•
Subjecting graphite to high pressures at ambient temperatures produces a carbon allotrope that has unique properties, which is known as superhard graphite.
•
The structure of this carbon allotrope has now been predicted by a theoretical technique known as Transition Path Sampling.
•
Researchers at Yale University have independently confirmed experimentally that the allotrope has the M-carbon structure.
CARBON EXISTS IN A NUMBER OF INTERESTING FORMS also known as allotropes. One of these forms, graphite, is pertinent to lubrication. It is a well-known solid lubricant that is well suitable for use in extreme applications in the presence of moisture up to a temperature of 650 C (
1).
Graphite is composed of a three-dimensional structure of carbon atoms arranged in a series of hexagons. It is a gray-black solid that can be used in such applications as brushes for electric motors.
There has been a good deal of research interest on a single layer of graphite occurring in two-dimensions, which is known as graphene. In a previous TLT article, graphene was found to be the strongest material ever examined (
2). In experiments where graphene films were indented until they broke, the break strength of this material was found to be 130 gigapascals, which is 200 times the strength of steel.
A third allotrope of carbon that is widely known to all of us is diamond. Diamond is the hardest natural substance known. Artem Oganov, professor in the department of geosciences and the department of physics and astronomy at the State University of New York at Stony Brook, says, “This thermodynamically stable allotrope of carbon can be formed from graphite under conditions of high pressures and temperatures (> 5 gigapascals and 927 C-2527 C).”
Almost 50 years ago, graphite was subjected to pressures above 15 to 19 gigapascals at ambient temperature. The result was the formation of another carbon allotrope that was distinctly different from diamond. Oganov says, “The result from this compression of graphite is a new form of carbon that is colorless, transparent and superhard. In fact, some researchers have designated this material as “superhard graphite.”
The results from this experiment have been replicated, but there has never been an elucidation of the structure of this carbon allotrope. A series of different structures have been proposed, but it remained unclear which one, if any, corresponds to the experimentally obtained material.
Oganov says, “The experimental observations made up to now can be explained by many of these structures. We initially proposed a three-dimensional structure that has four-coordinate carbon atoms (sp
3 hybridized), arranged in five-member and seven-member rings. The structure has monoclinic symmetry and has been designated as M-carbon. In our latest work, we proved that this is the only structure candidate that can correspond to the experimental observations.”
An image of M-carbon is shown in Figure 3. This allotrope of carbon has a physical hardness that is only slightly less than diamond. Oganov adds, “In certain directions, M-carbon can scratch diamond.”
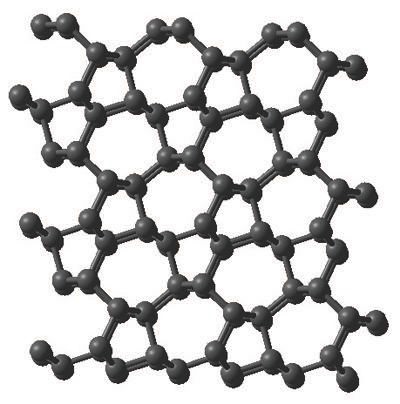 Figure 3. M-carbon has now been determined to be the correct structure for the carbon allotrope formed by compressing graphite under high pressure at ambient temperature. (Courtesy of the State University of New York at Stony Brook)
Figure 3. M-carbon has now been determined to be the correct structure for the carbon allotrope formed by compressing graphite under high pressure at ambient temperature. (Courtesy of the State University of New York at Stony Brook)
M-carbon only exists in a metastable state. Oganov explains, “When the pressure used to produce M-carbon from carbon is released, this allotrope will revert back to graphite at room temperature.”
The problem in structural determination is that researchers have only been able to collect low-resolution experimental data. For example, Oganov indicated that X-ray diffraction patterns were very broad because the sample of the M-carbon was mixed with graphite and very defective. A need exists to utilize higher-resolution experimental data to determine the actual structure of this carbon allotrope. This has now been done only very recently by Yale University researchers, whose work followed Oganov’s theoretical proof of the M-carbon structure.
TRANSITION PATH SAMPLING
Oganov and his fellow researchers used a novel technique known as Transition Path Sampling to predict that the structure of the carbon allotrope formed at room temperature under pressure is, in fact, M-carbon. He says, “The new allotrope was experimentally obtained by low-temperature compression of graphite. For this reason, the transformation product corresponds to the kinetically easiest phase, the one with the lowest activation barrier. Our task was to compare the potential pathways from graphite to all of the potential candidate structures. There were many candidate structures proposed over the last few years and designated by alphabet letters: M-, P-, Q-, R-, S-, W-, X-, Y-, Z-carbons, etc., the number totaling at least 40.”
Transition Path Sampling enabled the researchers to evaluate all of the potential transition paths and find the one exhibiting the lowest activation energy. Simulations unequivocally pointed at M-carbon as the kinetically easiest transformation product. Oganov and colleagues also directly compared the formation kinetics of Mcarbon and the other most prominent structures, bct4-carbon, W-carbon and diamond.
This remarkable result, as it turns out, has now been confirmed by a research group at Yale University, in a study that for the first time obtained high-resolution experimental data on this material. That experimental study also confirmed that the material has the M-carbon structure.
Future work will involve completing the experimental characterization of M-carbon. Oganov says, “Physical properties such as bulk modulus and shear modulus have been determined theoretically. We are now waiting for the experimental determinations.”
One of the challenges is to produce M-carbon samples with a high degree of perfection. Oganov says, “We believe that it is possible to produce a high quality M-carbon that should survive under ambient conditions once the pressure is released.”
Oganov is also looking to determine if other allotropes of carbon exist. He says, “There is a strong possibility that two different carbon allotropes can be produced from the two different forms of graphite, hexagonal and rhombohedral. So far, researchers only pressurized hexagonal graphite and obtained M-carbon. It remains unknown as to what will be found upon the compression of rhombohedral graphite.”
He also feels that many new allotropes can be created through compression of carbon nanotubes. As an element, carbon forms many different structures with other elements, which represent the basis for such areas as organic chemistry. The work done by Oganov is showing that carbon has multiple allotropes with different properties.
Additional information can be found in a recent article (
3) or by contacting Oganov at
artem.oganov@stonybrook.edu.
REFERENCES
1.
Gresham, R. (2003), “Solid Film Lubricants: Unique Products for Unique Lubrication,” TLT,
59 (10), pp. 28-31.
2.
Canter, N. (2009), “Graphene: The Strongest Material Ever Examined,” TLT,
65 (2), pp. 28-29.
3.
Boulfelfel, S., Oganov, A. and Leoni, S. (2012), “Understanding the Nature of ‘Superhard Graphite,’”
Scientific Reports,
2 (471), DOI: 10.1038/srep00471.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.