Effective NOx reduction without using precious metals
Dr. Neil Canter, Contributing Editor | TLT Tech Beat November 2012
A synthetic natural silicate mineral can reduce NOx emissions without the use of platinum.
KEY CONCEPTS
•
A synthetic version of the natural silicate mineral mullite has been found to effectively reduce NO
x emissions without the use of costly precious metals.
•
The synthetic mullite exhibits nitric oxide conversion rates that are 45% higher than platinum in emissions testing.
•
The key to the performance is the presence of a manganese dimer on the catalyst’s stepped surface.
RESEARCH CONTINUES TO FIND MORE COST-EFFICIENT ALTERNATIVES to the current diesel oxidation catalyst, NO
x treatment and diesel particulate filter used to reduce the levels of the main pollutants generated by a diesel engine.
This work is needed because the U.S. EPA has placed more restrictions on the level of these emissions that can be tolerated. For example, NO
x levels have been reduced from 4.0 g/bhp-hr (brake horse power per hour) to 0.2 g/ bhp-hr over the past decade.
In a previous TLT article, an approach to improve the effectiveness of the three-way catalyst involved using a direct-current (DC) plasma process to transform the precious metal starting materials into fine nanoparticles (
1). The researchers found that this technique led to superior performance over a longer operating period at a lower catalyst treat rate. As a result, an estimated 50% cost savings in precious metals can be realized.
But this approach and others does not eliminate the need for using precious metals. Kyeongjae (KJ) Cho, professor of materials science and engineering and physics at the University of Texas at Dallas, says, “Demand for platinum is increasing as more automobiles are produced globally. Supply has been a recent problem with a labor dispute in South Africa, leading to the price of platinum rising to over $1,470 per ounce.”
There is need for development of an effective catalyst that can reduce emissions but which isn’t based on platinum and other precious metals. Such a catalyst has now been developed.
MULLITE
Cho and his researchers at Nanostellar have developed an alternative catalyst based on the silicate mineral, mullite. He says, “In looking for an alternative catalyst, our work focused on evaluation of different oxide formulations. We have been looking at various metal oxides with different coordination numbers. Mullites were not the first series of candidates we looked at but have proven to be the most promising.”
Mullite is a natural silicate mineral discovered on the Scottish Isle of Mull in 1824. The researchers developed a synthetic version by replacing aluminum and silicon with samarium (a member of the lanthanide series) and manganese. Cho adds, “We prepared the catalyst through a co-precipitation technique.”
In this process, salts of the various metals (such as manganese nitrate, manganese carbonate, cesium carbonate, samarium oxide and strontium nitrate) were dissolved in deionized water with the assistance of the surfactant, PEG 600. The pH was adjusted to between 9 and 10 with tetramethylammonium hydroxide. Oxalic acid and hydrogen peroxide were then added to generate the synthetic mullite salts.
The best catalyst prepared is a combination of manganese, cerium, samarium and strontium oxides. It is known as MnCe-7:1, and the nomenclature reflects the stoichiometric ratio between manganese and cerium.
Cho says, “The initial mullite catalysts we prepared showed good performance, but there was need for improved thermal stability. MnCe-7:1 showed superior thermal and structural stability because of the addition of samarium and cerium.”
The researchers simulated the operating conditions of a diesel engine to evaluate several different mullite-based catalysts versus platinum. A reactant gas mixture containing 450 ppm of nitrous oxide and 10% oxygen gas was introduced, and the temperature was ramped up to 350 C and then ramped down. The oxidation of nitric oxide to nitrogen dioxide was measured, as this is the critical step for reducing NO
x and carbon soot emissions generated by the diesel engine.
The MnCe-7:1 catalyst displayed superior performance to platinum and the other mullite-based catalysts. Cho says, “All of the candidates were evaluated using equivalent catalyst loadings. We found that the MnCe-7:1 has nitric oxide conversion rates that are 45% higher than platinum. This mullite catalyst also displayed activity down to a temperature of 120 C.”
The researchers used a combination of analytical techniques and computer modeling to determine how MnCe-7:1 performs. Cho says, “We used a computational technique known as Density Functional Theory, which solves Schrödinger’s equation for the electrons in hundreds of atoms involved in the process.
Cho believes that the positioning of a manganese dimer on the stepped surface appears to be the key to the catalytic activity. Figure 1 shows an image of the MnCe-7:1 stepped surface. Oxygen, manganese and samarium are represented by red, purple and light green atoms, respectively.
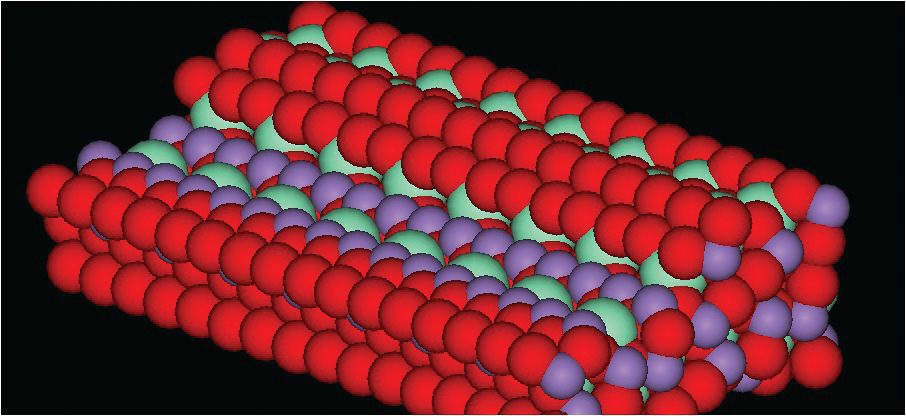 Figure 1. The stepped surface arrangement of the synthetic version of the natural silicate mineral, mullite is the key to its effectiveness as a catalyst in exhibiting a higher nitric oxide conversion rate than platinum in reducing NOx emissions. In this figure, the red, purple and light green atoms represent oxygen, manganese and samarium, respectively. (Courtesy of Nanostellar Inc.)
Figure 1. The stepped surface arrangement of the synthetic version of the natural silicate mineral, mullite is the key to its effectiveness as a catalyst in exhibiting a higher nitric oxide conversion rate than platinum in reducing NOx emissions. In this figure, the red, purple and light green atoms represent oxygen, manganese and samarium, respectively. (Courtesy of Nanostellar Inc.)
Cho maintains that the function of this mullite catalyst is just to reduce NO
x emissions. It has no effect on carbon monoxide or hydrocarbon emissions.
Further studies were done to show that MnCe-7:1 is compatible with the current commercial diesel oxidation and hydrocarbon oxidation catalysts. Cho says, “We added MnCe-7:1 on top of the diesel oxidation catalyst and found that it enhanced the conversion of nitric oxide to nitrogen dioxide, while not adversely affecting the reduction of carbon monoxide and hydrocarbon levels.”
From a lubricant perspective, Cho has not done any work to see if zinc dialkyldithiophosphate will negatively impact the MnCe-7:1 catalyst. He says, “The development of this mullite catalyst is just the first step in our long-term objective of developing a cheaper oxide catalyst that is also effective in reducing carbon monoxide and hydrocarbon emissions.” The startup company Nanostellar co-founded by Cho has been commercializing this technology under the brand name Noxicat.
Further information can be found in a recent reference (
2) or by contacting Cho at
kjcho@utdallas.edu.
REFERENCES
1.
Canter, N. (2009), “Nanoparticle-Based Emission Catalysts,” TLT,
65 (11), pp. 10-11.
2.
Wang, W., McCool, G., Kapur, N., Yuan, G, Shan, B., Nguyen, M., Graham, U., Davis, B., Jacobs, G., Cho, K. and Hao, X. (2012), “Mixed-Phase Oxide Catalyst Based on Mn-Mullite (Sm, Gd) Mn
2O
5 for NO Oxidation in Diesel Exhaust,”
Science,
337 (6096), pp. 832-835.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.