Determination of Free Boric Acid in Amine Borate Condensation Products by 11B NMR Spectroscopy
Introduction
Boric acid derivatives have been used in aqueous metalworking fluids for over thirty years. In addition to corrosion inhibition, borates provide buffering and reserve alkalinity. Recently the use of boric acid has come under the spotlight by the European Chemicals Agency.
The use of boric acid has not been restricted; however metalworking fluid companies marketing products in the European Union (EU) region need to know the level of free boric acid within additives in order to meet regulatory requirements. Afton Chemical has developed a method to determine very low levels of free boric acid in Afton Chemical’s boric acid derivatives marketed as Polartech® BA series.
EU Background: EU implementation of GHS means that products containing 5.5% or more of free boric acid need to be classified and labeled as Toxic to Reproduction.
Under the Annex II Safety Data Sheet requirements of REACH the presence of free boric acid also needs to be identified on the safety data sheet if it is contained in the product at a level 0.1% or more.
Other Countries: The EU CLP (GHS) classification, labeling and safety data sheet requirements for boric acid may subsequently have relevance with regard to the implementation of GHS in the USA, Canada and other countries.
Boric Acid: Boric acid is a weak Lewis acid with a pKa equal to 9.0 at 25°C and maximum solubility in water at only 5.5%. The boric acid will be present in two principle forms depending on the pH, for dilute solutions of boric acid below 0.025 molar in water.
At pH <5, boric acid B(OH)
3 is dominant and at pH > 12 the borate anion B(OH)
4- is dominant. The higher the pH, the greater the tendency to form the borate anion.
Afton Chemical’s boric acid derivatives are produced at a high temperature to ensure full reaction between boric acid and the alkanolamines generating a complex mixture of borate and polyborate species. The method Afton Chemical has developed is based on
11B NMR, capable of determining the free boric acid at a level of 0.1% in the POLARTECH® BA series.
11B NMR analysis is difficult in comparison to the more familiar 1H and 13C NMR, with a spin number of I=3/2 and a relative sensitivity approximately 4% that of 1H.
 Equation 1.
Equation 1.
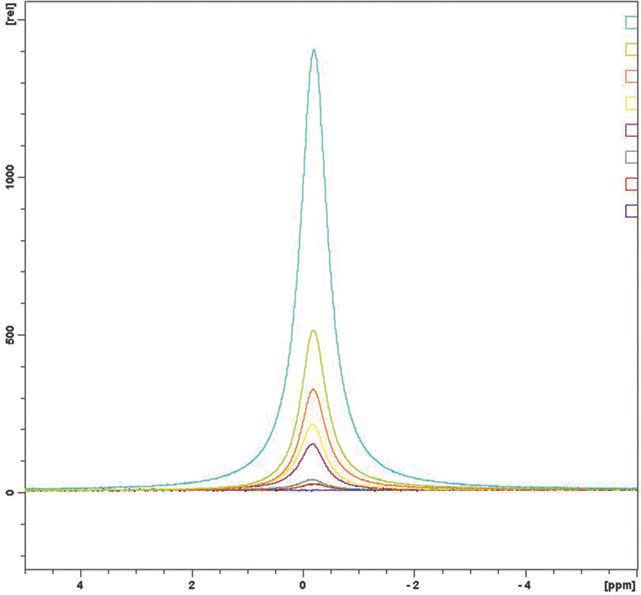
Figure 1. 11B NMR of boric acid solutions, aqua = 5% by weight, light green = 2%, brown = 1%, yellow = 0.5%, purple = 0.2%, green = 0.1%, red = 0.05% and blue = 0%.
NMR Method Development: A Bruker 400 MHz NMR spectrometer, capable of multi-nuclear analysis and equipped with a BBO probe was used. Ultra-low boron quartz tubes, Norrell® S-5-500-QTZ-7, were used to maximize the sensitivity towards
11B. The instrument was calibrated to detect very low levels of free boric acid.
Calibration was achieved by preparing boric acid in deuterated water [D
2O] at various concentrations from 5.0 to 0.05% by weight. Boric acid is set at 0 ppm chemical shift. Chemical shifts other than 0 ppm indicate the boron atom is chemically associated with other chemicals such as alkanolamine or present in more complex forms such as polyborate. With boric acid arbitrarily set at 0 ppm, this leads to other boron species with their own unique chemical shift having negative chemical shifts away from the boric acid.
A more detailed observation of boric acid in D
2O at calibration points at 2.0% and 0.05% are seen in
Figures 2 and 3.
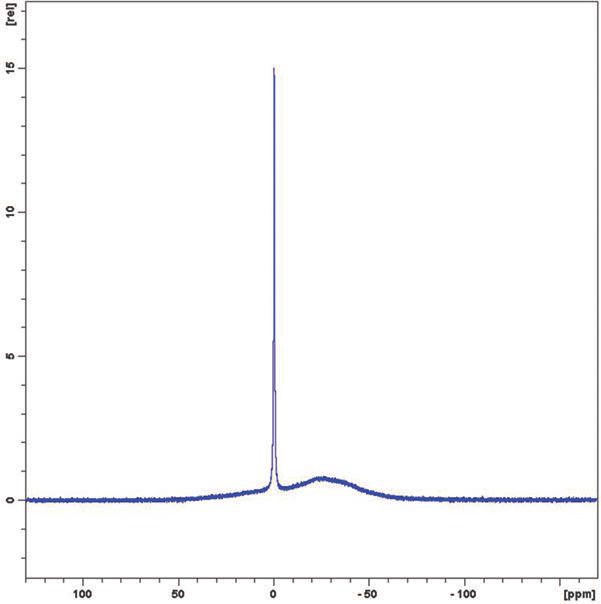 Figure 2. Boric acid in D2O at 2% by weight.
Figure 2. Boric acid in D2O at 2% by weight.
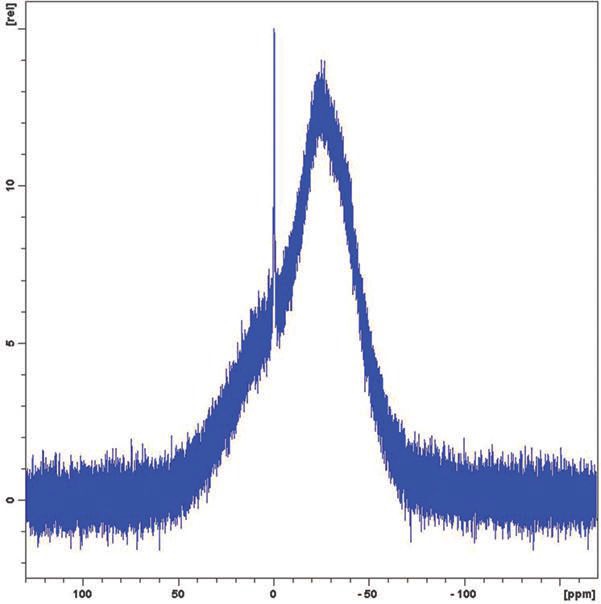
Figure 3. Boric acid in D2O at 0.05% by weight.
In both cases the free boric acid is clearly visible as the sharp peak at 0 ppm and the limit of detectability is to be below 0.02% by weight. The broad peak is borate anion and hydrated polyborate anions.
Additive Analysis: A laboratory reference product was prepared replicating typical manufacturing and QC conditions. The reference sample is indistinguishable from a typical production sample by normal QC analytical methods.
Dilution in D
2O improved resolution, enabling isolation of single species peaks. A 50% active dilution was used. Assessment of 10% dilution showed no sign of precipitation indicating the high hydrolytic stability of the borate condensates.
The lab products were based on boric acid and a 2% molar excess of monoethanolamine (MEA), to achieve an elemental boron content of 6%. The final product is a complex mixture of polyborate and mono borate species and unreacted MEA.
Analysis of the lab samples at 50% active in D
2O by
11B NMR gives strong peaks at -6, -10 and -13.5 ppm, a weak peak at -18.3 ppm and a broad tail of unresolved species between -13 to -20 ppm. No peak is visible at the reference 0 ppm suggesting that there is no boric acid or it is not detectable. To confirm the interpretation boric acid was spiked into the samples. The spiked samples showed strong peaks at 0 ppm and -18.3 ppm chemical shifts, indicating the presence of free boric acid and borate anion.
The technical work above established the analytical technique for accurately measuring free boric acid in laboratory samples and allowed Afton to evaluate its the full range of borate additives.
In each case key peaks were identified at -6.3, -10, -13.5, -15.4 and -18.3 ppm, with the relative peak heights varying dependent on the products formed. No evidence of any free boric acid at 0 ppm was observed.
Samples of the various borate additives were taken from recent plant retained production samples, all less than four weeks old. The analysis indicated no boric acid present demonstrating that the lab samples were truly representative of the plant materials.
Several typical formulations in the industry were prepared using a selection of borate condensates to evaluate if typical formulation would liberate boric acid. Typical metalworking fluid concentrates have between 10 to 40% active borate additives. The oil content was between 0 to 50% to cover the range of synthetic oil-free to medium oil semi-synthetic working fluids.
The same range of key NMR peaks at -6.3, -10, -13.5, -15.4 and -18.3 ppm were seen, with peak heights varying dependent on the additive used. We did observe in some formulations a small shift in the relative peak heights of these major peaks, which we attribute to interaction with the various acid and alkali components and possible complexation with hydroxyl species. Despite the complex nature of the formulations, no evidence of free boric acid at 0 ppm was seen.
Two representative borate condensate products at 4.8% & 5.6% elemental boron were selected to assess the long term stability of the borate condensates. Plant retained samples spanning 18 months, stored under typical warehouse conditions (temperature recorded were between -12 o C to +25 o C), were obtained.
Eighteen samples were analyzed and no evidence of free boric acid was seen at the 0 ppm chemical shift. In each case the sample is indicated in green, boric acid at 0.2% in red and boric acid at 0.1% in blue. In
Figures 4a & 4b and 5a & 5b, plant retained samples give essentially identical spectra whether 12 or 18 months old. This work concludes that the products, old and new, do not have any free boric acid proving their long term stability.
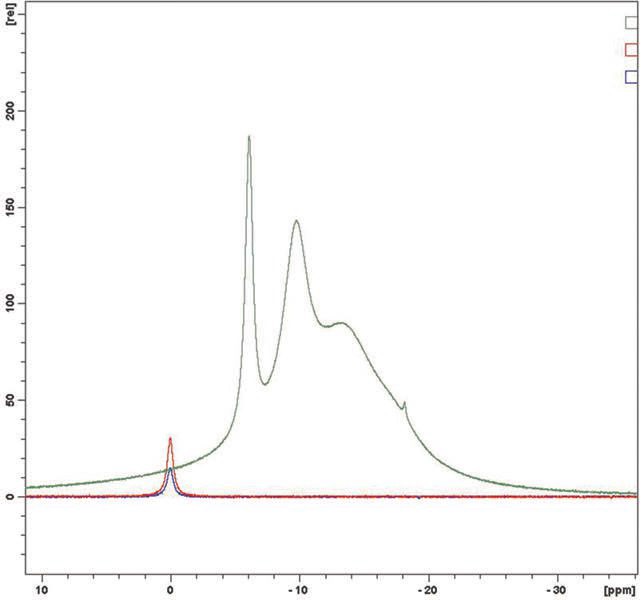 Figure 4a. Nov 2010
Figure 4a. Nov 2010
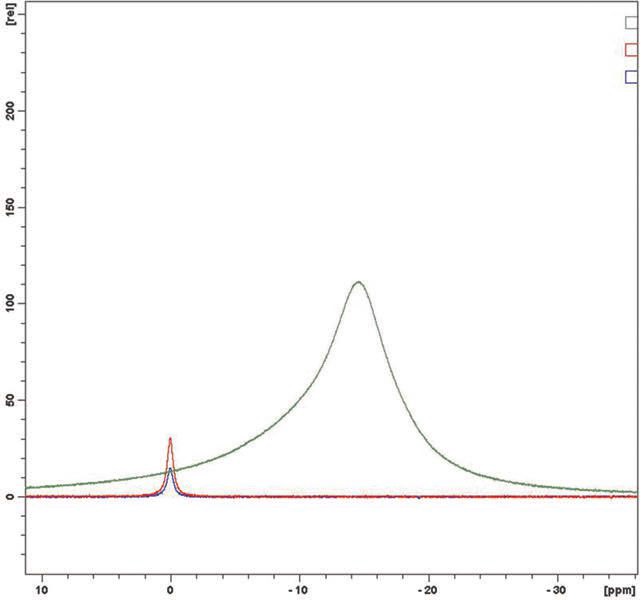
Figure 4b. April 2012
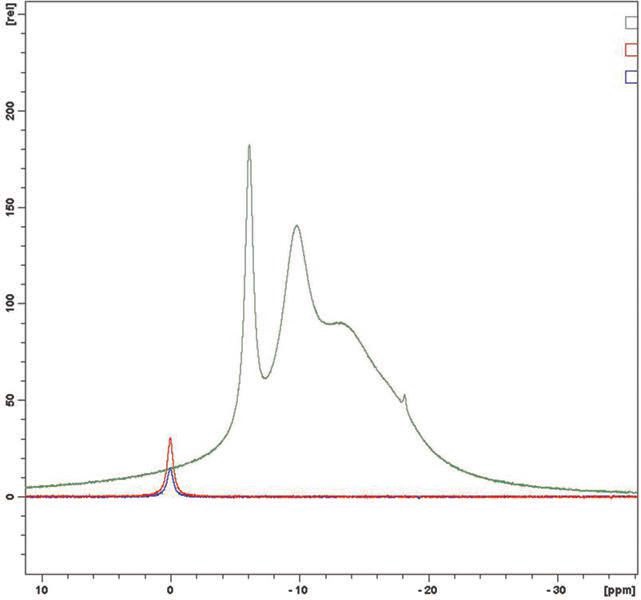
Figure 5a. April 2011
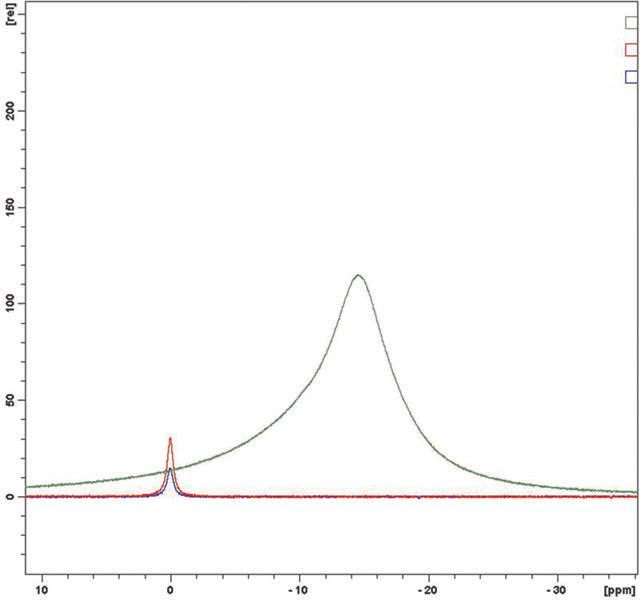
Figure 5b. March 2012
Developing this reliable method has been a remarkable technical achievement. Afton Chemical continues to investigate the use of borates and how they are used in different metalworking conditions.
Conclusion
Afton has developed a NMR method capable of identifying free boric acid below 0.02%. The boric acid condensation products that were analyzed showed no detectable level of free boric acid.
Over 50 individual borate condensate samples were analyzed, including plant and lab samples up to 18 months old and method development samples. We conclude that Afton Chemical’s POLARTECH® BA Series condensation products are free from detectable levels of boric acid (where the limit of detectability is 0.02% free boric acid).
Based on this work, and the evidence from formulated fluids, Afton Chemical is confident that Afton borate corrosion inhibitors, manufactured according to Afton’s processes used in aqueous MWF under normal working conditions and pH do not contain detectable levels of free boric acid. The supply and use of Polartech BA Series additives and metal working fluids incorporating them not required to carry labeling or notification regarding the presence of boric acid.