Synergy from combining two energy-generating technologies
Dr. Neil Canter, Contributing Editor | TLT Tech Beat July 2012
Both a microbial fuel cell and reverse electrodialysis have limitations in generating electricity.
KEY CONCEPTS
•
A microbial fuel cell and reverse electrodialysis are two techniques used to generate electricity that suffer from limitations.
•
Combining a microbial fuel cell with reverse electrodialysis creates a new device known as a microbial-reverse electrodialysis cell that more effectively treats waste and produces electricity.
•
Use of ammonium carbonate enables electricity to be generated and wastewater to be treated in virtually any region on the earth.
IN DEVELOPING AND UPGRADING LUBRICANTS, formulators are always looking to combine specific additive components to see how they complement each other. On the negative side, the concern is that components will act adversely toward each other, leading to formulation incompatibilities and inferior performance. But the hope is that finding the right components will lead to a performance level that is higher than what each can provide.
Two technologies that have been covered in TLT are fuel cells and wastewater treatment. One of the problems with fuel cells is the lack of durability of the main platinum catalysts used. In a previous TLT article, work was described that involved the development of a more stable catalyst consisted of a monolayer of platinum on palladium nanoparticles maintained on a porous carbon support (
1).
One of the most widely used approaches for treating water is reverse osmosis, which works well except in the case of removing anions, such as chloride, that can cause corrosion. In a previous TLT article, copper hydroxide ethanedisulfonate has been identified as a species with the ability to remove anions (
2).
A microbial fuel cell (MFC) is an electrochemical cell that utilizes the ability of bacteria to oxidize organic waste to generate electricity. Roland Cusick, a graduate student at Pennsylvania State University in State College, Pa., explains, “There are certain bacteria known as exoelectrogen that can oxidize organic matter at the anode, which leads to the transfer of electrons from the anode to the cathode where oxygen is reduced. The bacteria prefer volatile fatty acids such as acetic acid as a substrate. MFCs operate in a complex microbial community where other microbes are able to produce these fatty acids from organic waste.”
Reverse electrodialysis (RED) uses the ionic difference between fresh water and saltwater to produce electricity. Cusick says, “RED is a way to control the mix of fresh water and saltwater in between chambers of ion-selective membranes. Electricity is generated through alternating positive and negative ion-exchange membranes. Stacks of these membranes can be placed in series to generate a large voltage.”
Both the MFC and RED have limitations in generating electricity. Cusick says, “Both technologies are very costly for the amount of energy generated. MFCs also cannot be linked in series to boost power output. Besides inefficiency, REDs also suffer from electrode overpotentials and biofouling.”
A new approach has now been developed to combine the beneficial aspects of MFC and RED in order to generate a technology that more effectively treats waste while producing electricity at a much higher rate than either technology can do individually.
MRC
Cusick worked with Bruce Logan, Kappe professor of environmental engineering at Penn State, and Younggy Kim, a postdoctoral fellow, to combine MFC and RED in a device known as a microbial-reverse electrodialysis cell (MRC). He says, “We found that the MFC and RED were able to complement each other to produce a MRC that generated significantly more electricity.” Figure 3 shows an image of a MRC reactor.
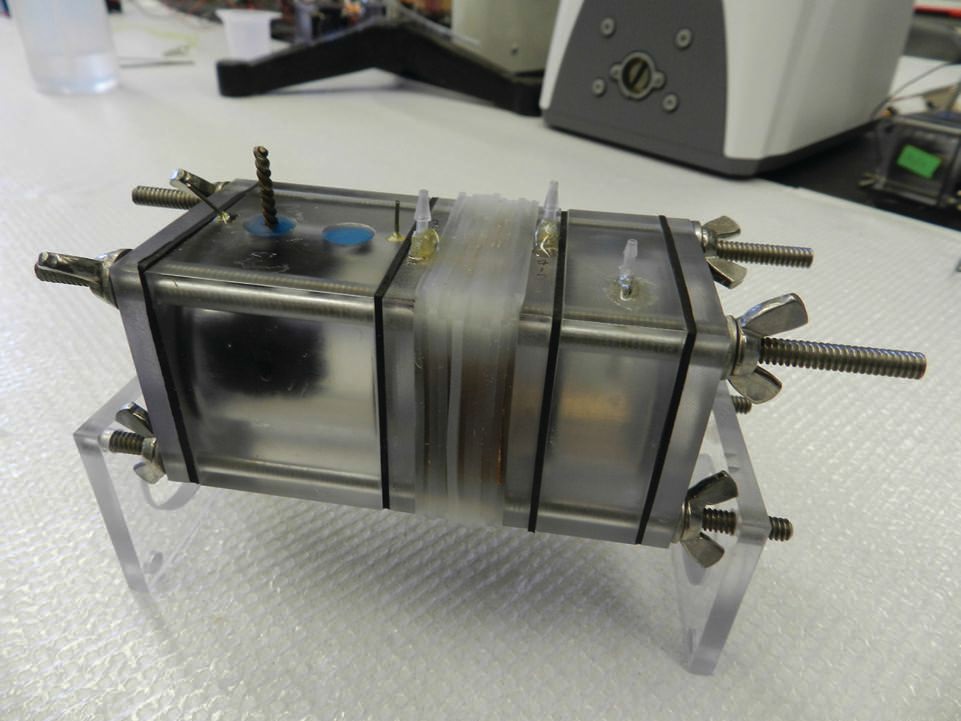 Figure 3. A microbial reverse-electrodialysis cell developed by combining two limited technologies known as a microbial fuel cell and reverse electrodialysis more effectively generates electricity. (Courtesy of Pennsylvania State University)
Figure 3. A microbial reverse-electrodialysis cell developed by combining two limited technologies known as a microbial fuel cell and reverse electrodialysis more effectively generates electricity. (Courtesy of Pennsylvania State University)
The slow kinetics and low voltage produced by a MFC were increased through the use of the salinity-driven potential contributed by the RED. This enabled the bacteria to enhance the amount of energy they produced from organic waste.
The initial MRC used synthetic seawater, which was a 600 mM solution of sodium chloride and river water prepared from mixing sodium chloride with deionized water at salinity ratios of 100, 50 and 1. A RED stack with 10 cells was used in which the five seawater and five riverwater cells were sandwiched between anode and cathode chambers.
One issue faced with the use of sodium chloride as the electrolyte was the decrease in the pH in the anolyte from 7 to 5.5 and the increase in the pH of the catholyte to 11.8. Cusick says, “The pH drop was due to the generation of hydrogen ions resulting from the oxidation of the organic waste by the bacteria. In the case of the catholyte, the pH increase is due to consumption of the hydrogen ions.”
One problem with this approach is that it is limited to being used in coastal areas where there is ready access to salt and fresh water needed for the MRC. A new approach is to use a thermolytic solution, such as ammonium bicarbonate, that can be continuously regenerated with waste heat greater than 40 C.
Cusick says, “We are familiar with ammonium bicarbonate because it is used in the desalination technique known as forward osmosis. Substitution of ammonium bicarbonate for sodium chloride in the anode and cathode chambers making up the RED stack has been found to produce 5.6 watts per square meter of electricity.”
The benefit of using ammonium bicarbonate is that its two components (ammonia and carbon dioxide) can be readily boiled out of the solution, recaptured and reused. Cusick says, “Our technology provides an incentive for energy-poor areas to produce electrical power from wastewater. The additional benefit is that water sanitation will also be achieved.”
Future work will involve using MRC for the generation of hydrogen. Cusick indicates that the efficiency of the process needs to be improved through the use of cheaper membranes that can operate in series. Additional information can be obtained from a recent article (
3) or by contacting Logan at
blogan@psu.edu.
REFERENCES
1.
Canter, N. (2011), “Stable Fuel Catalyst,” TLT,
67 (2), pp. 12-13.
2.
Canter, N. (2011), “Water Treatment of Anions,” TLT,
67 (12), pp. 12-13.
3.
Kim, Y. and Logan, B. (2011), “Microbial Reverse Electrodialysis Cells for Synergistically Enhanced Power Production,”
Environmental Science & Technology,
45 (13), pp. 5834-5839.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.