More effective zeolite nanocatalysts and membranes based on layers
Dr. Neil Canter, Contributing Editor | TLT Tech Beat April 2012
These structures were prepared using a solid-state exfoliation process with a polymer.
KEY CONCEPTS
•
Stable zeolite nanosheets were prepared by using a solid-state exfoliation process involving the production of a polystyrene nanocomposite.
•
Two nanosheet structure types knowN as MWW and MFI were produced that have typical dimensions of 0.5 micron x 0.5 micron x 3 nanometers.
•
The zeolite nanosheets demonstrated the ability to act as a filter in separating orthoxylene from paraxylene.
CATALYSTS PLAY AN IMPORTANT ROLE IN FACILITATING the processing of base oils, synthetic lubricant basestocks and additives in reducing emissions. Much attention has been paid to developing nanocatalysts that show the potential to provide even better performance because of their smaller sizes and larger surface areas.
In a previous TLT article, a nanocatalyst was prepared by impregnating iron nanoparticles into porous carbon microspheres (
1). Most of the nanoparticles have diameters less than 20 nanometers. The iron-based nanocatalyst very effectively reduced aqueous hexavalent chromium. Future work will determine if it can be used in multipollutant applications such as removing NO
x from automotive emission streams.
Zeolites are a well-known catalyst that have been used as adsorbents and catalysts for several decades. One of their leading uses is as a catalyst in refinery applications such as hydrocracking.
But preparation of suitable zeolite nanocatalysts is difficult. Michael Tsapatsis, professor of chemical engineering and materials science at the University of Minnesota in Minneapolis, says, “We have been interested in developing zeolite nanocatalysts that can act as thin films to be used in such applications as filtration. The problem is that repeated attempts to develop such nanomaterials led to either destruction of the crystal structure initially formed or aggregation of the layers.”
According to Tsapatsis, the ideal structure contains thin, two-dimensional planes that are anisotropic. He adds, “A thin layer can help both as a filter and as a catalyst. In the former case, diffusion time through a filter can be reduced. For the latter, the thin layer can expedite the movement of molecules, thereby speeding up a reaction.”
Ultimately, Tsapatsis is looking for the sweet spot where the layer structure can be preserved at the nanoscale without causing the crystalline structure to be destroyed. A process has now been developed to achieve this goal.
NANOSHEETS
Tsapatsis and his associates have developed a procedure for preparing stable zeolite nanocatalysts. The zeolite is present in the form of crystal-type nanosheets, as shown in Figure 2.
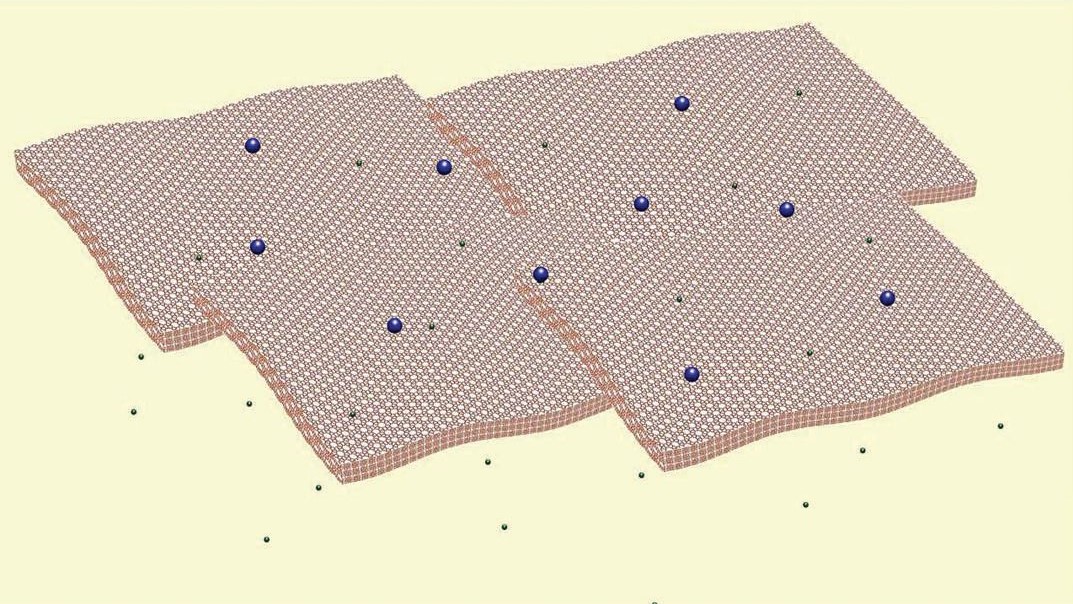 Figure 2. The two types of stable, zeolite nanosheets prepared through a solid exfoliation process show potential as catalysts and filters to separate isomers of organic compounds. (Courtesy of the University of Minnesota)
Figure 2. The two types of stable, zeolite nanosheets prepared through a solid exfoliation process show potential as catalysts and filters to separate isomers of organic compounds. (Courtesy of the University of Minnesota)
Tsapatsis says, “We prepared the nanosheets using a solid-state exfoliation process with a polymer. Our preference would have been to use a solvent, but the method simply did not work because the nanosheet structures were destroyed.”
Instead, the researchers treated the zeolite precursor with polystyrene, exhibiting a weight-average molecular weight of 45,000 in a melt blend. This procedure was conducted under a nitrogen atmosphere in a co-rotating, twin screw extruder. Temperatures between 150 C and 170 C were used in this step of the process.
The resulting nanosheet-polystyrene nanocomposite was then added to toluene and sonicated in order to dissolve the polymer and suspend the nanosheets. Centrifuging was then done to separate and filter the larger particles.
After isolating the zeolite nanosheets, high-temperature heating (typically 500 C) was done to remove any residual organics by combustion.
Tsapatsis says, “The formation of an intermediate nanocomposite with polystyrene works quite well. Unfortunately, this process takes one day, which makes it very tedious and lengthy.”
Future work by the researchers will be done to find a more efficient method. Tsapatsis is hopeful that a better solvent system can be found.
The researchers produced nanosheets of the MWW and MFI structural types. They have typical dimensions of 0.5 micron x 0.5 micron x 3 nanometers.
Tsapatsis explains, “These two structure types have different pore sizes controlling transport across the zeolite nanosheet layers. For MWW, the smaller aperture along the thin dimension of the layers has a diameter of 3 angstroms, which means that this structure type can allow only small molecules such as hydrogen to cross. In the case of MFI, the pore has a diameter of 5.5 angstroms, which enables the zeolite nanosheets to act as a filter to separate organic compounds such as para-, ortho- and metaxylene.”
The zeolite nanosheets were analyzed by transmission electron microscopy, atomic force microscopy and x-ray diffraction. They can be used to coat porous substrates to form highly packed and oriented coatings.
Testing was done to separate orthoxylene from paraxylene. At temperatures up to 200 C, the two xylene isomers were incorporated into a nitrogen or argon gas stream and passed through a membrane containing the zeolite nanosheets. Tsapatsis says, “We obtained good separation of the xylene isomers. The flux and selectivity were high.”
Besides improving the process, future work will involve evaluating the zeolite nanosheets under higher temperatures and pressures that are more reflective of commercial processing conditions. Liquid feeds also need to be used.
Tsapatsis says, “We envision determining if these zeolite nanosheets can work with other chemicals such as separating linear versus branched hydrocarbons.”
The high cost of separating substances (estimated to be 15% of the total energy consumed) means there is great potential for the use of zeolite nanosheets. The researchers have filed a provisional patent and are looking for partners to further develop and commercialize this technology.
Further information can be found in a recent paper (
2) or by contacting Tsapatsis at
tsapatsis@umn.edu.
REFERENCES
1.
Canter, N. (2011), “Iron-Based Nanocatalyst,” TLT,
67 (4), pp. 12-13.
2.
Varoon, K., Zhang, X., Elyassi, B., Brewer, D., Gettel, M., Kumar, S., Lee, J., Maheshwari, S., Mittal, A., Sung, C., Cococcioni, M., Francis, L, McCormick, A., Mkhoyan, K. and Tsapatsis, M. (2011), “Dispersible Exfoliated Zeolite Nanosheets and Their Application as a Selective Membrane,”
Science,
334 (6052), pp. 72-75.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.