A new type of ultra-stable oil adsorbent
Dr. Neil Canter, Contributing Editor | TLT Tech Beat March 2012
Greater stability may facilitate use in real-world applications.
KEY CONCEPTS
•
A new type of metal-organic framework (MOF) has been developed based on perfluorinated groups and is known as FMOFs.
•
FMOFs are more stable in the presence of air, humidity, water and sunlight.
•
This new class of molecules adsorbs between 190 and 300 kilograms per cubic meters of organic hydrocarbons and aromatics, which represents nearly the best-known performance for any MOF while rejecting water.
THE IMPACT OF OIL SPILLS IS SEEN BY ALL OF US WORLDWIDE. In 2010 cleanup of oil spills was estimated to be over $10 billion. For the lubricant industry, base oil remains the most important raw material used, and there remains a good chance that we will have to continue to deal with oil spills.
In a previous TLT article, a new type of technology to clean up oil spills was introduced that has fewer limitations than the conventional dispersants, polymers and sorbents currently used (
1). A green solidifier derived from a sugar-based derivative can gelate oil even in the presence of water. At elevated temperatures, the gelation can be reversed so that the oil can be recovered and the green solidifier reused.
One class of molecules that has been found to act as effective adsorbents is metal-organic frameworks (MOFs). In a previous TLT article, the potential to use MOFs to adsorb gases such as carbon dioxide was discussed (
2). MOFs are three-dimensional crystalline structures that contain inorganic units joined together by organic linkers. They have the potential to remove automotive combustion byproducts from the emissions stream.
Dr. Mohammad Omary, professor of chemistry at the University of North Texas in Denton, Texas, says, “MOFs have enormous potential as adsorbents, but most of them lack stability under ambient conditions in the presence of air, humidity, water and sunlight.” These limitations restrict the ability to use MOFs in open-air applications.
There is a need for a new type of MOF that exhibits superior stability so that it can be used in real-world applications. Such a new class of molecules has now been developed.
FMOFs
Omary and his researchers have developed a much more stable MOF type that contains perfluorinated (such as trifluoromethyl) groups and is known as fluorous metal-organic frameworks (FMOFs). He says, “The fluorinated characteristics of FMOFs produce extraordinary stability to water, humidity, air and light. In addition, the hydrophobic, fluorine-lined pore surface offers more favorable adsorption properties such as high volumetric capacity, particularly for hydrocarbons under real-world conditions that might include water and humid air.”
The FMOFs evaluated by the researchers are prepared from silver (I) and 3,5-bis(trifluoromethyl)-1,2,4-triazolate. Omary says, “The silver atoms are coordinated to the fluorous triazolate organic ligands.”
Omary became interested in these molecules through work he was doing in the area of molecular electronics. He adds, “In the work we were doing with thin film conductivity, we became involved in evaluating compositions with silver triazolate derivatives which are organic semiconductors. After evaluating these derivatives, we noted that they exhibit a high surface area and are very stable.”
Initial work with the FMOFs involved evaluating their ability to store hydrogen. Omary says, “The FMOFs have high adsorption density to effectively store hydrogen under high pressure but only at cryogenic temperatures.”
The researchers initially studied the ability of FMOFs to uptake water vapor in ambient temperature under relative humidity conditions ranging from 0% to 100%. The FMOFs pick up little to no water even in a completely water-saturated environment. As a comparison, the researchers evaluated the hydrophilic material, Zeolite-5A and a hydrophobic-activated carbon known as BPL carbon.
As expected, Zeolite-5A adsorbs water at a relatively low humidity level of 10%. BPL carbon rapidly picked up water but only when the relative humidity rose to above 40%.
One other experiment run to highlight the stability of the FMOFs was to soak a single crystal in water for several days. Omary says, “After soaking in water, the x-ray crystal structure was identical to a FMOF that was not treated with water. All other classes of MOFs will normally break down in the presence of water.”
This water-rejecting characteristic of FMOFs suggests that they may be superhydrophobic. Omary comments, “We really expect FMOFs to be superhydrophobic but are still working to prove it. Future work will involve obtaining exact contact angle measurements, which need to be done by placing the FMOFs on a flat surface.”
A similar hydrocarbon uptake experiment was conducted with FMOFs in the presence of hydrocarbons that are typical ingredients of gasoline such as n-hexane and cyclohexane and aromatic molecules such as benzene, toluene and para-xylene. The FMOFs rapidly adsorb all of these hydrophobic molecules at low relative pressures.
Omary says, “We found that the FMOFs adsorbed between 190 and 300 kilograms per cubic meters of the organic molecules, which represents nearly the best known performance for any MOF. The process can be reversed by heating the FMOF to a temperature of 120 C under vacuum conditions.”
The adsorption process is so stable that the researchers were able to obtain a crystal structure for a FMOF that adsorbed six molecules of toluene per macroporous channel and three molecules in each of the six surrounding nanoporous cages (
see Figure 3). Omary indicates that the effective adsorption of hydrocarbons and aromatics is due to a combination of hydrophobicity and capillary action.
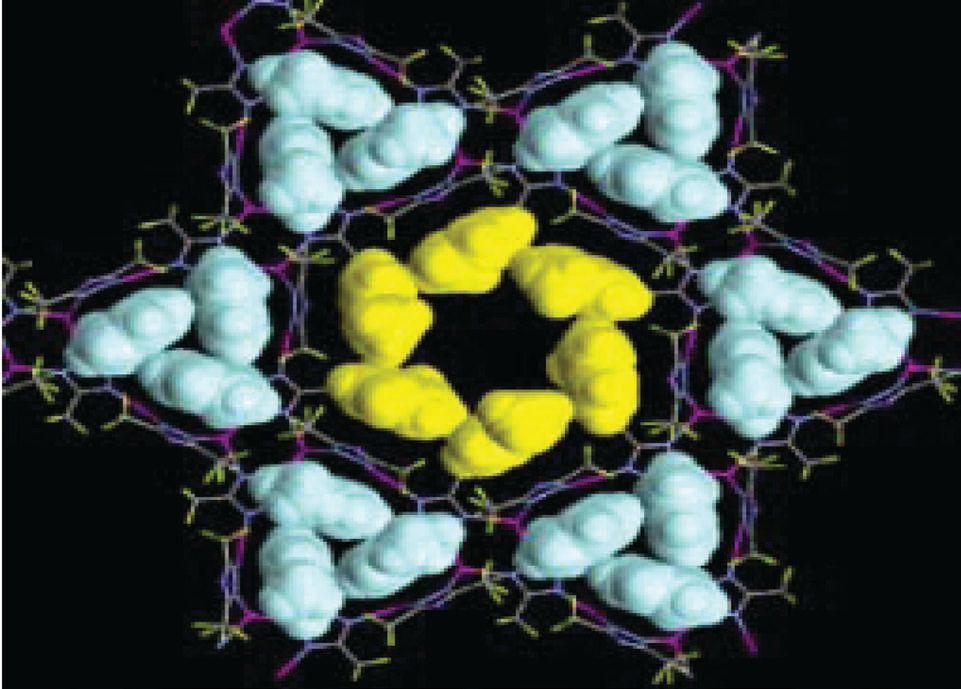 Figure 3. This crystal structure shows how readily FMOFs adsorb aromatic molecules such as toluene. Six molecules of toluene (shown in yellow) are adsorbed in the macroporous channel, while three molecules of toluene (shown in blue) are adsorbed in each of the six surrounding nanoporous cages. (Courtesy of the University of North Texas)
Figure 3. This crystal structure shows how readily FMOFs adsorb aromatic molecules such as toluene. Six molecules of toluene (shown in yellow) are adsorbed in the macroporous channel, while three molecules of toluene (shown in blue) are adsorbed in each of the six surrounding nanoporous cages. (Courtesy of the University of North Texas)
Future work will involve evaluating the ability of FMOFs to adsorb larger molecules such as anthracene and pentacene for further advances in molecular electronics, as well as multicomponent mixtures for many industrial applications. Omary says, “We are very interested in examining how FMOFs adsorb complex hydrocarbon blends, including lubricants.”
Additional information can be found in a recent article (
3) or by contacting Omary at omary@unt.edu.
REFERENCES
1.
Canter, N. (2006), “MOFs: More Effective Gas Adsorbers,” TLT,
62 (4), pp. 12-15.
2.
Canter, N. (2010), “A Green Approach to Cleaning Oil Spills,” TLT,
66 (12), pp. 14-15.
3.
Yang, C., Kaipa, U., Mather, Q., Wang, X., Nesterov, V., Venero, A. and Omary, M. (2011), “Fluorous Metal-Organic Frameworks with Superior Adsorption and Hydrophobic Properties toward Oil Spill Cleanup and Hydrocarbon Storage,”
Journal of the American Chemical Society,
133 (45), pp. 18094-18097.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.