Self-lubricating omniphobic surface
Dr. Neil Canter, Contributing Editor | TLT Tech Beat January 2012
Researchers develop a synthetic material that is repellent to oil and water.
KEY CONCEPTS
•
A new material known as SLIPS repels both oils and water.
•
SLIPS is prepared by impregnating a perfluorinated lubricant in a porous network of polytetrafluoroethylene nanofibers.
•
A variety of non-polar hydrocarbons such as mineral oil and water-based fluids such as salts, acids and bases were all found to be repelled.
ONE OF THE MAIN PROBLEMS IN WORKING WITH SURFACES is figuring out a way to minimize the presence of contaminants. Water is an important contaminant that impacts most lubrication systems. Several TLT articles have discussed the goal of developing a super-hydrophobic surface that would repel water. One of the articles describes how researchers have detected the presence of small air bubbles that contribute to the extreme water repellency of a super-hydrophobic surface (
1).
A second article describes work done to develop a surface that repels ice (
2). The key feature was taking the fluorinated silicon material used to prepare the surface and fabricating it into various geometries such as honeycombs.
Nature has developed several ways to prepare hydrophilic and hydrophobic surfaces that can effectively repel specific materials. Dr. Tak Sing Wong, Postdoctoral Fellow in the School of Engineering and Applied Sciences at Harvard University in Cambridge, Mass., says, “The lotus leaf is a good example of a surface that repels water. In the lotus effect, water is supported on a thin layer of air trapped by the textured structure of the leaf. This enables the water to roll off the leaf.”
Wong explains that the problem with this approach, unfortunately, is that the layer of air is very unstable, which enables water to penetrate and be trapped on the microstructure of the leaf at slightly elevated pressure. A second problem is that liquids with lower surface tensions than water (such as ethanol and hexane) can penetrate into the texture easily. This effect means the surface loses repellency.
A second plant that has a more effective repellent surface is the
Nepenthes pitcher plant. Wong says, “The reason this surface is better at repelling materials is that water acts as a slippery liquid to repel immiscible materials impinging on the surface of the plant. As a result, an ant that has oils on its feet will slide right off the surface of the plant.”
But the pitcher plant has limited capabilities in repelling materials other than ant oils. Inspired by these examples from nature, a synthetic surface that is truly repellent to most materials has now been developed.
SLIPS
Wong, Sung Hoon Kang, a graduate student in the School of Engineering and Applied Surfaces at Harvard University, and other researchers working with Dr. Joanna Aizenberg, Amy Smith Berylson Professor of Materials Science at Harvard University, have developed materials known as slippery liquid- infused porous surfaces, also known as SLIPS.
SLIPS are prepared by impregnating a lubricating liquid that is oil and water repellent in a porous network of polytetrafluoroethylene nanofibers, as shown in Figure 1. The lubricating liquids used were perfluorinated and include such examples as 3M’s Fluorinert FC-70 and DuPont’s Krytox oils.
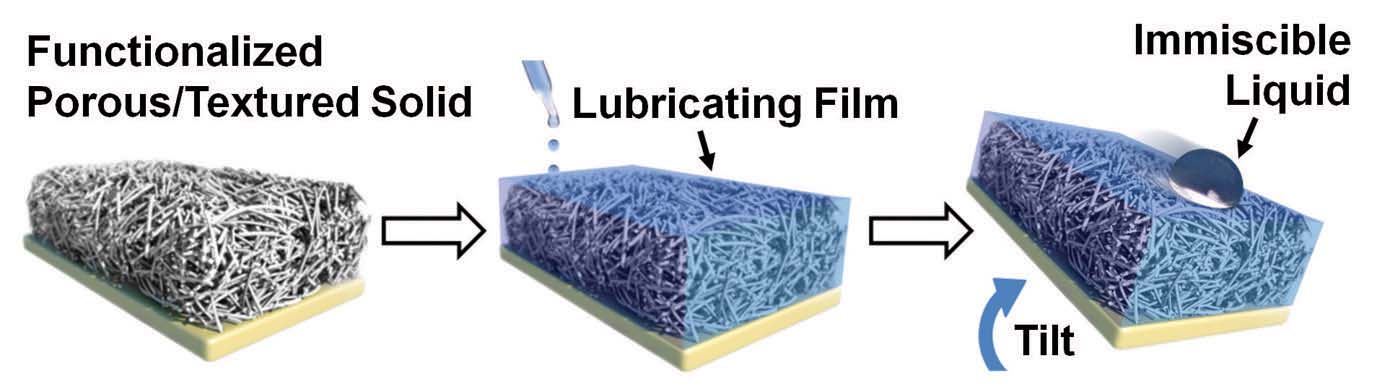 Figure 1. An omniphobic surface that repels both oils and water is prepared by impregnating a lubricating liquid in a porous network of polytetrafluoroethylene nanofibers. (Courtesy of Harvard University)
Figure 1. An omniphobic surface that repels both oils and water is prepared by impregnating a lubricating liquid in a porous network of polytetrafluoroethylene nanofibers. (Courtesy of Harvard University)
Kang explains the criteria used in preparing SLIPS. He says, “The first parameter is that the lubricating liquid should not be miscible with the liquid that is being repelled. A second criterion is that the liquid used in the lubricating layer must exhibit better wetting on the surface of the nanofiber solid than the liquid being repelled.”
The key performance feature is the layer of lubricating fluid that forms on the surface of the nanofiber solid to ensure that all materials interacting with the surface will fall off rapidly. Surface roughness or texture is important to ensure that the liquid lubricating layer forms a nice film. Kang says, “The texture helps to hold the lubricating layer inside the solid and then enables the liquid to form a nice film. Without the roughness, the lubricating layer will only form a droplet on the surface.”
The researchers conducted tilting angle and contact angle hysteresis analysis to evaluate the ability of SLIPS to repel liquids. Kang says, “The former analysis involves placing a drop of the liquid to be tested on the surface and then tilting the surface until the drop slides away. In the latter, contact angle hysteresis measures the difference between the maximum downhill contact angle and the minimum uphill contact angle of the surface as it is tilted. The lower the tilting angle and the contact angle hysteresis, the better SLIPS can repel liquids.”
A variety of different water- and oil-based liquids were tested. Wong says, “From the water-based standpoint, we evaluated salt solutions such as sodium chloride, acids such as hydrochloric acid and bases such as sodium hydroxide. Nonpolar hydrocarbons such as mineral oil, octane and hexadecane were also tested as were polar organic liquids such as ethanol.”
In fact, Wong mentioned that the researchers also evaluated a commercially available vacuum pump oil in this study. Every liquid tried was repelled by SLIPS, which means that this technology exhibits omniphobic characteristics.
The only liquid that SLIPS will not repel is fluorinated materials. Kang explains: “Fluorinated materials are miscible with the lubricating layer, which means they cannot be repelled.”
One other very interesting aspect about SLIPS is that the lubricating film can act as a self-healing coating. Kang says, “If structural damage occurs in the porous material, then the liquid layer very quickly fills the voids in a self-healing process.”
There are a large number of potential applications for SLIPS. The researchers believe that their technology can be used in applications such as oil transport, self-healing coatings and antifouling coatings for vessels. Wong adds, “SLIPS form transparent coatings, so there is potential for using them in optical applications such as solar cells and also in insect-repellent surfaces.”
Future work entails evaluating the ability of SLIPS to function under extreme temperature conditions and over long operating periods. Wong says, “We are testing the ability of SLIPS to operate at temperatures over 200 C and at temperatures below -30 C. We also are looking to optimize the combination of lubricating liquid and porous structures to increase the operating lifetime of the omniphobic material.
Additional information can be found in a recent article (
3) or by contacting Dr. Tak-Sing Wong at
tswong@seas.harvard.edu.
REFERENCES
1.
Canter, N. (2010), “Presence of Nanobubbles on Superhydrophobic Surfaces,” TLT,
66 (8), pp. 14-15.
2.
Canter, N. (2011), “Ice Repellent Surfaces,” TLT,
67 (2), pp 12-15.
3.
Wong, T., Kang. S., Tang, S., Smythe, E., Hatton, B., Grinthal, A. and Aizenberg, J. (2011), “Bioinspired Self-Repairing Slippery Surfaces with Pressure-Stable Omniphobicity,”
Nature,
477 (7365), pp. 443-447.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.