Water treatment of anions
Dr. Neil Canter, Contributing Editor | TLT Tech Beat December 2011
Researchers have developed a copper-based material that potentially can remove anions from water.
KEY CONCEPTS
•
Removal of anions such as chlorides from effluent streams has not been effective.
•
A new compound known as copper hydroxide ethanedisulfonate shows promise in the removal of dicarboxylates and metal oxo anions such as permanganate.
•
Copper hydroxide ethanedisulfonate possibly can be used with a cation exchange resin to remove both harmful anions and cations from water prior to use or prior to effluent discharge.
Water is a critically important resource that needs to be (1.) purified prior to use in a manufacturing plant and (2.) waste treated effectively to meet the requirements of the regulatory authorities in the region where the plant is located. For purification of water, several methods are available for use that differ in effectiveness.
The technique of reverse osmosis (RO) is most reliable in ensuring consistent water quality. A previous TLT article discussed a new technology that improves the performance of the RO filter membrane (
1). The researchers modified the polyamide membrane with zeolite molecular-sieve nanoparticles. As a result, the RO membrane becomes more hydrophilic, which improves performance and reduces the chances of fouling.
RO and other techniques have been fairly effective at removing cations such as calcium and magnesium from effluent streams. The problem has been dealing with anions such as chloride that can readily cause corrosion. A previous TLT article discussed a new approach for chelating chlorides reversibly using an aryl-triazole foldamer (
2). This material binds chloride under visible light and releases it when exposed to ultraviolet light.
For the past 12 years, Scott Oliver, associate professor of chemistry at the University of California-Santa Cruz in Santa Cruz, Calif., has conducted research to find more effective materials that trap pollutants. He says, “In existing membranes, sodium ions are readily available to be exchanged with cations in the water, but no negatively charged zeolites have been developed to effectively exchange anions with a nontoxic negatively charged species. Our objective has been to move in the opposite direction of negatively charged zeolites by looking to prepare a positively charged material.”
One approach that has been looked at is to work with layered double hydroxides (LDHs) also known as hydrotalcites. Oliver says, “These species are known as clays, occur widely in nature and are easy to synthesize. They contain two-dimensional, extended architectures in which the cationic layers are held together by anions in-between them.”
LDHs are able to exchange anions, but Oliver indicates that a complete swap is not possible. The main problem is their inability to completely exchange due to carbonate, which is prevalent because carbon dioxide is present in water.
A need exists for a more effective material to effectively exchange anions. Such a material has now been developed.
SLUG-26
Oliver, in collaboration with University of California graduate student Honghan Fei, has developed a copper-based species that has a two-dimensional layered structure with a strong ability to exchange anions. The anion coordinated to the copper hydroxide layers is 1,2-ethanedisulfonate.
The material is known as copper hydroxide ethanedisulfonate and is designated as SLUG-26, which stands for University of California-Santa Cruz, Structure No. 26. Synchrotron single-crystal X-ray diffraction analysis was conducted to determine the structure of this compound.
Copper hydroxide ethanedisulfonate contains a copper hydroxide cationic layer that contains two copper atoms in a two-dimensional structure. Between the copper hydroxide cationic layers is the ethanedisulfonates, which provide charge-balance. Figure 3 shows a schematic of the structure.
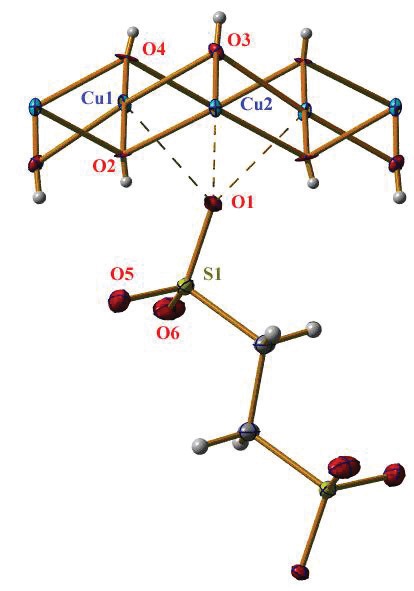 Figure 3. Copper hydroxide ethanedisulfonate is a newly developed material that has the potential to effectively remove anions from water. (Courtesy of the University of California-Santa Cruz)
Figure 3. Copper hydroxide ethanedisulfonate is a newly developed material that has the potential to effectively remove anions from water. (Courtesy of the University of California-Santa Cruz)
Oliver says, “This work is the culmination of a long project in which we looked at various chemical species including those based on bismuth. We looked at a number of anions but found that ethanedisulfonate provided a good template for the structure.”
The synthesis of the copper hydroxide ethanedisulfonate is highly temperature dependent. Oliver says, “We prepared the copper hydroxide ethanedisulfonate under hydrothermal conditions at a temperature of 150 C. Too high a temperature (between 160 C and 180 C) leads to the formation of copper oxide as the main species. At temperatures below 125 C, the reaction occurred at a very low rate.”
The researchers evaluated the efficacy of the copper hydroxide ethanedisulfonate with various dicarboxylates such as malonates, succinates and glutarates. The exchange rates for these three dicarboxylates ranged from 92.4% to 100% under room temperature conditions.
Other species that were evaluated include metal oxo anions such as permanganate. Oliver says, “We looked at permanganate because it is a good model for pertechnetate, which is a byproduct of nuclear waste and can leach out into groundwater.”
As measured by ultraviolet/visible light spectroscopy, a 48-hour experiment at room temperature showed a dramatic decrease in permanganate concentration. In contrast, a LDH known as magnesium aluminum hydroxycarbonate exhibited only a fifth of the ability to capture permanganate anions.
Oliver has not determined the mechanism for how copper hydroxide ethanedisulfonate conducts the anion exchange. He says, “We believe that the openness of the structure enables an effective exchange of anions between the copper hydroxide layers.”
Oliver has not evaluated the ability of the copper hydroxide ethanedisulfonate to trap chloride anions and other halogens. Studies have also yet to be done to see how this material can be recharged.
Future work will involve seeing if a cation less expensive than copper can be found without sacrificing performance. Oliver says, “A material such as SLUG-26 can be used in series with a cation exchange resin to remove both harmful anions and cations from water prior to use or prior to effluent discharge.”
Additional information can be found in a recent paper (
3) or by contacting Oliver at
soliver@ucsc.edu.
REFERENCES
1.
Canter, N. (2007), “Revolutionary Reverse Osmosis Membrane,” TLT,
63 (8), pp. 14–16.
2.
Canter, N. (2011), “Chloride Binder,” TLT,
67 (1), pp. 12–13.
3.
Fei, H. and Oliver, R. (2011), “Copper Hydroxide Ethanedisulfonate: A Cationic Inorganic Layered Material for High-Capacity Anion Exchange,”
Angewandte Chemie International Edition,
50 (39), pp. 9066–9070.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.