Enzymatic production of renewable fuels
Dr. Neil Canter, Contributing Editor | TLT Tech Beat October 2011
A bacterial biosynthetic pathway could possibly be used to manufacture petroleum-based fuels.
KEY CONCEPTS
•
A biosynthetic pathway used by bacteria to produce long chain monounsaturated olefins possibly could be used to manufacture petroleum-based fuels.
•
The mechanism of the first step of the pathway in which the enzyme OleA catalyzes the condensation of two Coenzyme A fatty acyl groups had not been clearly determined.
•
Reaction of fatty acyl groups with various chain lengths with OleA produced a beta-keto fatty acid intermediate.
Interest in finding an efficient way to manufacture renewable fuels from biological precursors continues to be an active area of research. A large number of options exist because nature provides a number of synthetic pathways for producing hydrocarbons that are precursors to fuels.
This work differs in part from research underway to develop biofuels. In a previous TLT article, a study comparing how the combustion of biofuels compares to hydrocarbon-based fuels was discussed (
1). Biodiesel combustion is much less complex than the pathway for petroleum-derived diesel. There are lower concentrations of toxic species seen with petroleum-derived diesel, but biodiesel does produce significant levels of oxygenated toxic species such as aldehydes due to the presence of oxygen.
One interesting biosynthetic pathway used by bacteria is the synthesis of long chain monounsaturated olefins (C23 to C33) through a mechanism known as head-to-head condensation of fatty acyl groups. These species then can be converted into petroleum-based fuels.
Lawrence Wackett, Distinguished McKnight Professor of Biochemistry at the University of Minnesota in St Paul, Minn., says, “Fatty acids in living cells are typically tethered to the sulfur-based protein groups present in Coenzyme A in order to render them more soluble in water. In the head-to-head condensation process, two fatty Coenzyme A fatty acyl groups are combined in the first step of the biosynthetic pathway. Several additional steps then take place to produce the final product, which is a long chain olefin.”
The enzyme to catalyze the first step of the process is known as OleA. Of equal importance is this enzyme is present in 70 bacterial strains. The result is that there are a large number of organisms that can possibly manufacture olefins through this biosynthetic pathway.
There is controversy about the mechanism of the initial step. Two options involve the condensation of two acyl thioesters or a decarboxylation process that combines a beta-keto ester with an acyl thioester.
Research has just been conducted to determine the mechanism of the first step and to gain a better understanding of how to use this biosynthetic pathway to produce long chain olefins.
OVEREXPRESSION OF OLEA
The researchers cloned the genes used to manufacture OleA from the bacterium
Xanthomonas campestris and overexpressed them into
E. coli. At this point, the E. coli was grown on a large scale in a 550-liter bioreactor.
Cells containing OleA were disrupted after passing through a chilled French pressure cell at 1,200 psi and then centrifuged. OleA was isolated by using fast protein liquid chromatography.
Various fatty acyl groups ranging in chain length from C8 to C18 were reacted with OleA. Reaction products identified by GC-MS were a combination of ketones and fatty acids. The former were prepared by condensation of two of the fatty acyl groups while the latter are hydrolysis products.
Wackett says, “We found that ketone production was best achieved with chains between C12 and C14 as compared to either C8 or C18. This occurred because the middle chains were better able to bind to the hydrophobic region of the enzyme.”
The ketones were formed because the beta-keto fatty acid intermediate is highly reactive and can readily decarboxylate. A critical element was to determine if this process occurs enzymatically, which would mean that it is part of the biosynthetic pathway or else if it happens because of the highly reactive nature of the beta-keto fatty acid intermediate. The latter can readily decarboxylate.
Testing using a
14C version of an acyl thioester indicates that ketone formation is due to the highly reactive nature of the intermediate over time and not an enzymatic process. Further work compared the beta-keto fatty acid prepared from the OleA enzymatic process and from a synthetic pathway. No difference in structure was seen based on GC-MS analysis.
Two enzymes known as OleC and OleD catalyze two ensuing steps to produce the long chain olefin. Wackett explains, “The beta-keto fatty acid produced from OleA is reduced to an alcohol acid by OleD. Further processing by OleC leads to decarboxylation of one equivalent of carbon dioxide and elimination of the alcohol to form the olefin.”
The researchers proved that the beta-keto fatty acid is the intermediate by treating a specific compound of this class with OleC and OleD. This experiment resulted in the synthesis of the expected long chain olefin.
A reaction mechanism was proposed by the researchers in which OleA generates a beta-keto fatty acid by facilitating the condensation of two acyl thioesters. Future work will entail expanding the evaluation to other bacteria strains.
Wackett says, “We envision evaluating this biosynthetic pathway in other bacteria strains to assess its effectiveness.”
In taking a broader perspective, Wackett believes this finding helps in the goal of developing renewable fuels from bacteria, sunlight and carbon dioxide. He says, “We are ultimately working to use carbon dioxide in combination with sunlight to facilitate the formation of sugars in bacteria by photosynthesis. Sugars then can act as an intermediate to eventually form acyl thioesters that can be used to produce the long chain olefins.
Figure 3 shows a fluorescent microscope image of the cells grown by the researchers to produce the long chain olefins from carbon dioxide. Photosynthetic bacteria that convert carbon dioxide into sugars are stained red. Bacteria used to convert sugars into olefins are stained blue.
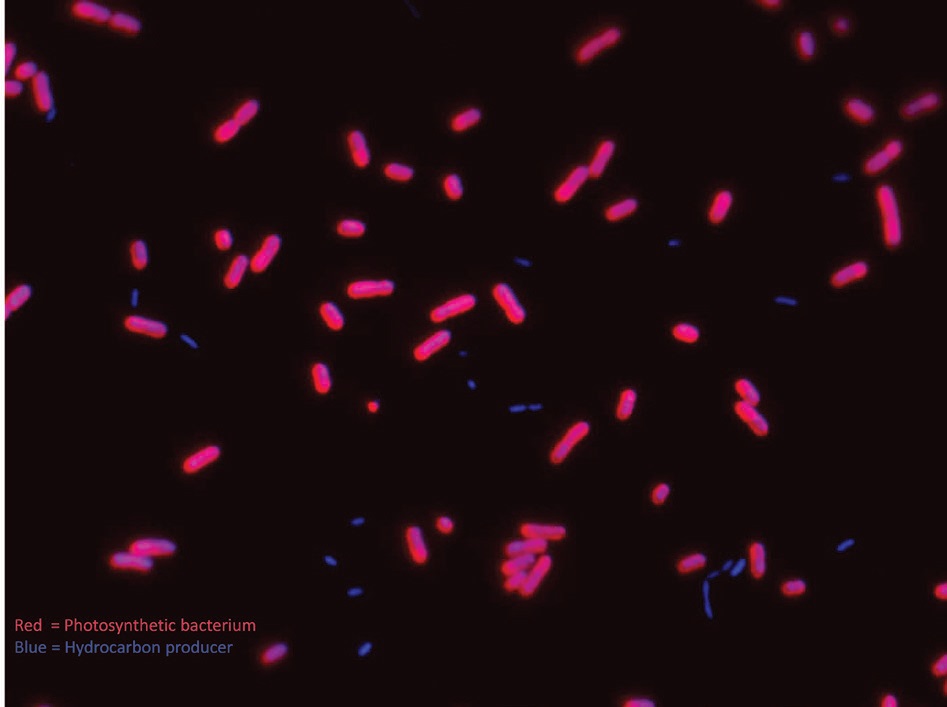 Figure 3. Determination of the mechanism for the conversion of sugars to long chain olefins by bacteria stained blue will help facilitate the goal of developing renewable fuels from bacteria, sunlight and carbon dioxide. The bacteria stained red convert carbon dioxide into sugars. (Courtesy of Neissa Pinzon and Kelly Aukema/University of Minnesota)
Figure 3. Determination of the mechanism for the conversion of sugars to long chain olefins by bacteria stained blue will help facilitate the goal of developing renewable fuels from bacteria, sunlight and carbon dioxide. The bacteria stained red convert carbon dioxide into sugars. (Courtesy of Neissa Pinzon and Kelly Aukema/University of Minnesota)
Further information on the reaction catalyzed by OleA can be found in a recent article (
2) or by contacting Wackett at
wacke003@umn.edu.
REFERENCES
1.
Canter, N. (2011), “Combustion of Biofuels,” TLT,
67 (3), pp. 14–15.
2.
Frias, J., Richman, J., Erickson,J. and Wackett, L., (2011), “Purification and Characterization of OleA from Xanthomonas campestris and Demonstration of a Non-decarboxylative Claisen Condensation Reaction,”
Journal of Biological Chemistry,
286 (13), pp. 10930–10938.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.