Spinning molecules
Dr. Neil Canter, Contributing Editor | TLT Tech Beat July 2011
A new computational study determines how molecules rotate.
KEY CONCEPTS
•
A computational study has determined more about how molecules rotate on a gold surface.
•
The symmetry of the gold atoms on the surface affects molecular rotation.
•
Two other critical factors influencing rotation are the geometry of the molecule and how strongly it binds to the gold surface.
Gaining a better understanding of how molecules operate on surfaces has implications for developing better lubricants. One other factor is the growing interest in preparing machines such as microelectromechanical systems that operate on the nanoscale.
In a previous TLT article, research that led to the synthesis of nanocars and nanotrucks was described (
1). Complicated molecules that were approximately 3-by-4 nanometers in size were prepared in multiple-stage synthesis processes. The cars were placed on a gold surface and heated to 170 C to initiate movement.
One very important phenomenon that molecules might perform on surfaces is that of rotation. Species that spin and rotate while attached to a surface are known as molecular rotors. Anatoly Kolomeisky, associate professor of chemistry at Rice University in Houston, says, “Surface-mounted molecular rotors represent a promising class of artificial devices readily useful in nanotechnology. These rotors can be easily controlled and manipulated to perform specific tasks by themselves or in conjunction with other nanoscale devices.”
In earlier work, Kolomeisky noted that experimental evidence was seen for dialkyl sulfies rotating on a gold surface. Kolomeisky says, “We saw scanning tunneling microscope images of these molecules in motion as heat was applied. This motion occurred because the images went from linear to rectangular to hexagonal as the temperature increased.”
An alkylated sulfide positioned on a gold surface is a good system to evaluate because sulfur has a strong affinity for gold and is positioned between two alkyl chains. This enables the alkyl groups to freely rotate around the sulfur atom. Dialkyl sulfides are also of interest because they are used as extreme pressure additives in lubricant applications.
Kolomeisky believes that further determination of what factors affect the ability of dialkyl sulfides and other similar species rotate can lead to the development of more effective molecular rotors. Such a study has now been conducted.
COMPUTATIONAL ANALYSIS
In an extension of the work done on nanocars, Kolomeisky and his graduate student, Alexey Akimov, have utilized a series of computational analyzes to learn more about how molecules rotate. Kolomeisky says, “We used a simple computational model in which we adjusted the chains attached to the sulfur atom and also worked with different types of chains.”
Two of the factors evaluated were molecular symmetry and surface symmetry. The assumption might be made that extending the alkylchain might make it more difficult for rotation to occur. But computational analysis shows that this is not always the case. Kolomeisky explains, “Increasing the chain length of a symmetric dialkyl sulfide does lead to more interactions with the gold surface, which should cause the rotation to slow down. This is compensated for by increased steric repulsion and chain flexibility, so no effect is seen. The steric repulsion means that the interaction with each carbon atom is smaller. The net result is extending the chain of a symmetric dialkyl sulfide does not affect the rate of rotation.”
If the dialkyl sulfide chains are asymmetric, then the computational analysis shows the chains will rotate slower than for symmetric chains. The researchers tested two dialkyl sulfides with the same number of carbon atoms except that Kolomeisky says, “Our simulations found that asymmetric chains will rotate slower because the carbon atoms on the longer chain will have to expend additional energy to overcome rotational barriers.”
The symmetry of the gold atoms also plays a role in influencing the rate of molecular rotation. The researchers evaluated how symmetric dialkyl sulfides rotate on gold (100) and gold (111) surfaces. Initial work started with the simplest example, dimethyl sulfide.
For the gold (100) surface, the methyl groups of each chain are exposed to similar local surface geometries. They can be on top of the gold surface atoms where the repulsion is strongest or on bridges connecting gold surface atoms where repulsions are weaker.
In the gold (111) surface, the methyl groups are never in the same surface environment. This means that the chances for both methyl groups being on top of a gold surface atom where the rotational barrier is highest are lower than in the gold (100) surface.
As a result, dimethyl sulfides rotate faster on the gold (111) surface than on the gold (100) surface. This characteristic is also seen as the number of carbon atoms in the alkyl chains rises.
The researchers analyzed a different type of molecule that contains a series of methylene groups sandwiched between a monocyclopentadienyl iron complex on one end and a ferrocene group on the other end. In this molecule, the former complex has a strong interaction with the gold surface while the rest of the chain with the ferrocene group is free to rotate. Figure 2 shows an image of this molecule on the gold surface.
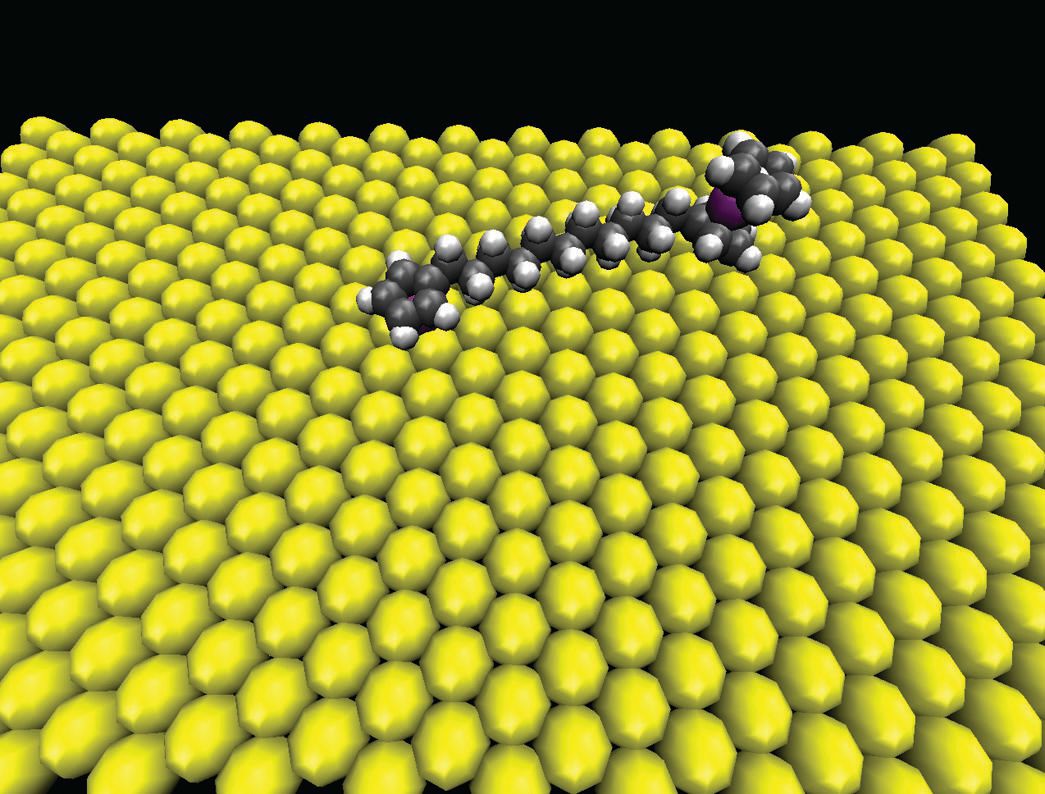 Figure 2. Molecular symmetry and surface symmetry are two of the parameters that influence the ability of molecules that contain a series of methylene groups sandwiched between a monocyclopentadienyl iron complex on one end and a ferrocene group on the other end to rotate on a gold surface. (Courtesy of Rice University)
Figure 2. Molecular symmetry and surface symmetry are two of the parameters that influence the ability of molecules that contain a series of methylene groups sandwiched between a monocyclopentadienyl iron complex on one end and a ferrocene group on the other end to rotate on a gold surface. (Courtesy of Rice University)
The researchers found that the computational analysis provided a different result than with the dialkyl sulfides. An increase in the carbon chain length leads to reduced rotation as higher barriers are present to restrict motion.
Two factors are critical in influencing molecular rotation on the gold surface. These are the ease of molecular rotation due to the geometry of the molecules and how strongly the molecule binds to the gold surface.
Future research will turn to evaluating more than one molecular rotor on the gold surface. Kolomeisky says, “We want to see how the two molecules interact with the gold surface and with each other. Does 1+1 necessarily equal 2? We do not believe so.”
Additional information can be found in a recent reference (
2) or by contacting Kolomeisky at
tolya@rice.edu.
REFERENCES
1.
Canter, N. (2006), “Developing a Motorized Nanocar,” TLT,
62 (8), pp. 10–11.
2.
Akimov, A. and Kolomeisky, A. (2011), “Dynamics of Single-Molecule Rotations on Surfaces that Depend on Symmetry, Interactions and Molecular Sizes,”
The Journal of Physical Chemistry C,
115 (1), pp. 125–131.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.