Methane: Potential Liquid Fuel Precursor
Dr. Neil Canter, Contributing Editor | TLT Tech Beat June 2011
An entirely new process uses a stable palladium (III) complex to facilitate the conversion of methane to ethane.
KEY CONCEPTS
•
A catalyst has been found to facilitate the conversion of methane to ethane in an unprecedented process.
•
The mechanism of the process is almost certainly radical in nature.
•
This process can be used to expedite the conversion of methane to higher molecular weight hydrocarbons that could be useful as liquid fuels.
Fuels and components in lubricants are mainly organic molecules derived from natural or petroleum sources. Synthesis of these species usually involves reaction processes from organic precursors.
In a previous TLT article, a new synthetic technique was described to derivatize aromatic compounds such as benzene, xylene and cumene (
1). This reaction technique may lead to a more efficient process for preparing such lubricant additives as antioxidants and detergents.
One objective for synthetic organic chemists is to develop procedures for taking the smallest organic molecule—methane, which is widely available as the main component in natural gas—and finding a way to easily produce higher molecular weight hydrocarbons that could be useful as liquid fuels.
Past efforts involved the use of the Fischer-Tropsch process to take syngas (a mixture of carbon monoxide and hydrogen) and convert it to synthetic basestocks. The problem is that this process is inefficient.
In searching for ways to find more efficient synthetic pathways, researchers have been working with transition metal complexes that are known as good catalysts. Liviu Mirica, assistant professor of chemistry at Washington University in St. Louis, Mo., and his research group were working with a palladium (II) compound that showed promise to catalyze the splitting of water. Mirica says, “The catalyst worked only adequately, but we did notice that it could be easily oxidized, even in the presence of oxygen in air.”
But the palladium (II) compound showed sufficient potential in this electrochemical process for Mirica to use it as a catalyst for preparing ethane from methane. Such a process was unprecedented until now.
PALLADIUM (III) COMPLEX
Mirica and his research group have developed a stable palladium (III) complex that can facilitate the preparation of ethane from methane. He says, “Palladium chemistry is a perfect way to facilitate this process because we have been able to take a palladium (II) complex that can coordinate four donor atoms in a coplanar arrangement and oxidize it to a tetragonally distorted octahedral palladium (III) complex that is hexacoordinate.”
Organometallic palladium (III) complexes have been proposed to act as good catalysts for converting C-H bonds into C-C couplings in an oxidative manner. However, until now, no mononuclear Pd (III) complexes have been isolated and characterized.
Mirica says, “We oxidized a palladium (II) complex prepared with the tetradentate ligand N4 using controlled potential electrolysis to generate the stabilized palladium (III) complex. The key reason for the stability of the palladium (III) complex is that the N4 ligand has two arms that swing above and below the palladium atom.”
The palladium (III) complex is quite stable in the solid state at a temperature of -20 C and is stable in a solution with specific organic solvents for a few weeks at room temperature in the absence of light. Crystal structures of the palladium (III) complex were prepared showing that the four nitrogen atoms of N4 are coordinated to the palladium atom. This leaves the palladium (III) complex with two additional sites to coordinate species that can be reacted together in a catalytic fashion.
Exposure of a palladium (III) N4 complex with methyl groups in the fifth and sixth sites to visible light at room temperature leads to the formation of ethane. Mirica says, “This process represents the first time that a palladium complex has been used to generate ethane from monomethyl complexes.”
The researchers were also able to use the corresponding phenyl complex to generate biphenyl as a reaction product. Figure 2 shows a schematic of the reaction process, the crystal structure for the stable palladium (III) complex and the structure of N4.
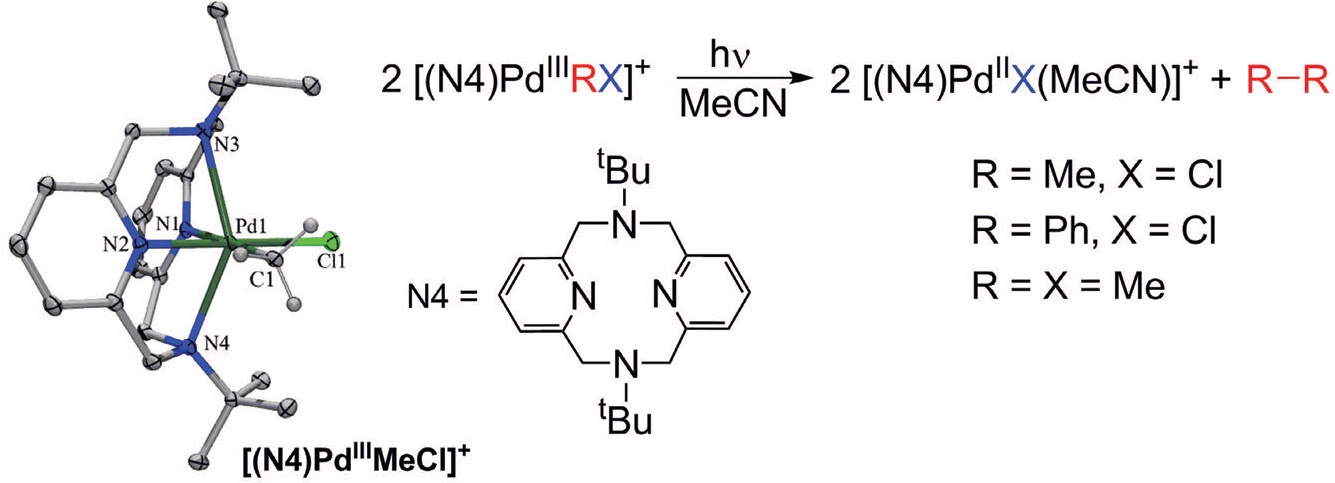 Figure 2. The unprecedented reaction process shown may prove to be a step in the process of converting methane to higher molecular weight hydrocarbons potentially useful as liquid fuels. (Courtesy of Washington University)
Figure 2. The unprecedented reaction process shown may prove to be a step in the process of converting methane to higher molecular weight hydrocarbons potentially useful as liquid fuels. (Courtesy of Washington University)
Mirica says, “This process appears to be flexible enough that other chemical species coordinated to the palladium atom can be used to prepare longer-chain hydrocarbons. It is certainly conceivable that a methane group could be catalyzed in a series of steps to generate eventually a higher molecular weight species such as butane or even octane.”
In effect, the palladium (III) complex is catalyzing the oxidation of methane to ethane. The CPE process uses a potentiostat to remove electrons from the starting material through the use of electric current. Mirica says, “CPE is the quickest way to remove electrons but has the disadvantage of being lengthy.”
Mirica indicates that the researchers found ferrocenium salts work as a chemical oxidizing agent. He adds, “We have determined in recent work that even oxygen from the air is an effective oxidant. Our reactions can now be run by exposing the palladium (II) complex to one atmosphere of oxygen gas at room temperature.”
Mirica mentions that a halogen lamp with a regular light bulb is the only light source needed. He says, “Medium intensity light is only required, and we have seen that laboratory light is suitable for driving the reaction.”
The mechanism of this process is almost certainly radical in nature, according to Mirica. Alkyl radical scavengers such as TEMPO completely prevent the formation of ethane.
Future research is now directed to see if the proposed palladium catalyst can be used to break one of the C-H Bonds in methane. This would be the first step in the targeted catalytic process of converting methane to ethane.
Mirica says, “We are now running reactions with methane gas under fairly high pressure (20 to 30 atmospheres) and monitoring the reaction by using nuclear magnetic resonance spectroscopy.
The mild conditions make this process potentially very attractive to commercialize. Additionally, Mirica’s research goals included the development of a process to efficiently reverse the combustion process by turning carbon dioxide into hydrocarbon molecules suitable as fuel sources.
Additional information on this research can be found in a recent article (
2) and by contacting Mirica at
mirica@wustl.edu.
REFERENCES
1.
Canter, N. (2010), “Facile Olefination of Aromatic Derivatives,” TLT,
66 (3), pp. 14–15.
2.
Khusnutdinova, J., Rath, N. and Mirica, L. (2010), “Stable Mononuclear Organometallic Pd (III) Complexes and Their C-C Bond Formation Reactivity,”
J. Am. Chem. Soc.,
132 (21), pp. 7303–7305.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.