Examining corrosion and oxidation at the nanoscale
Dr. Neil Canter, Contributing Editor | TLT Tech Beat April 2011
An electrochemical cell takes measurements as an accessory for the atomic force microscope.
KEY CONCEPTS
•
An electrochemical cell is used with an atomic force microscope to take oxidation and corrosion measurements at the nanoscale.
•
This instrument conducts electrochemical experiments and develops images of the changes occurring on a surface at the same time.
•
The electrochemical cell can accommodate samples of various sizes. A typical sample is one centimeter in diameter and a few millimeters thick.
The main function of a lubricant is to minimize friction and wear as two surfaces move against each other. In this process, a lubricant interacts with the atoms on the surface of both materials.
We have gained a pretty good understanding of both friction and wear on the macroscale. Further insight is being obtained at the nanoscale through the use of the atomic force microscope (AFM).
A previous TLT article discussed research to determine the frictional effects of small numbers of atomic layers at the nanoscale (
1). The AFM was used in a technique known as friction force microscopy to measure frictional forces detected with atomically thin sheets of materials such as graphene.
This study concluded that a single sheet of atoms produces more friction than multiple sheets of atoms. The reason is that a single sheet more readily bends to conform to the AFM tip, which causes more contact area and, as a consequence, higher friction.
Minimization of corrosion and oxidation are key objectives in maintaining the operating life of a lubricant at an optimal level. Both phenomena have their origins at the molecular level.
An instrument that can be used to evaluate the corrosion and oxidation of surfaces at the nanoscale would be invaluable in helping to better understand both processes. Such an instrument is now available.
ELECTROCHEMICAL CELL
Oxidation and corrosion measurements can be made through the use of an electrochemical cell that is now available as an accessory for the AFM. The electrochemical cell or EC Cell is available for the MFP-3D AFM from Asylum Research in Santa Barbara, Calif.
Dr. Maarten Rutgers, staff scientist for Asylum Research, says, “We developed the electrochemical cell in collaboration with professor Richard Compton of the chemistry department at the University of Oxford (U.K.). Some of their research focuses on the electrochemical deposition of materials resulting from driving a current between surfaces immersed in electrolyte. Traditionally, the deposits are studied by removing the sample from the electrolyte and placing it in an electron microscope down the hall.”
The purpose of the AFM with the electrochemical cell is to conduct the electrochemical experiments and develop images of the changes occurring at the same time. This saves a considerable amount of research time and allows observation of many processes as they occur.
The electrochemical cell works both in liquid and in air. Rutgers says, “Current can be cycled through the cell and voltage can be measured. At the same time, an image of what is occurring can be obtained.”
A photo of the electrochemical cell is shown in Figure 2. This device is a container made from the inert polymer, polyetheretherketone. All components which can potentially come into contact with fluid are made of inert materials. This includes FKM or Klarez O-rings. A window into the cell is made from quartz.
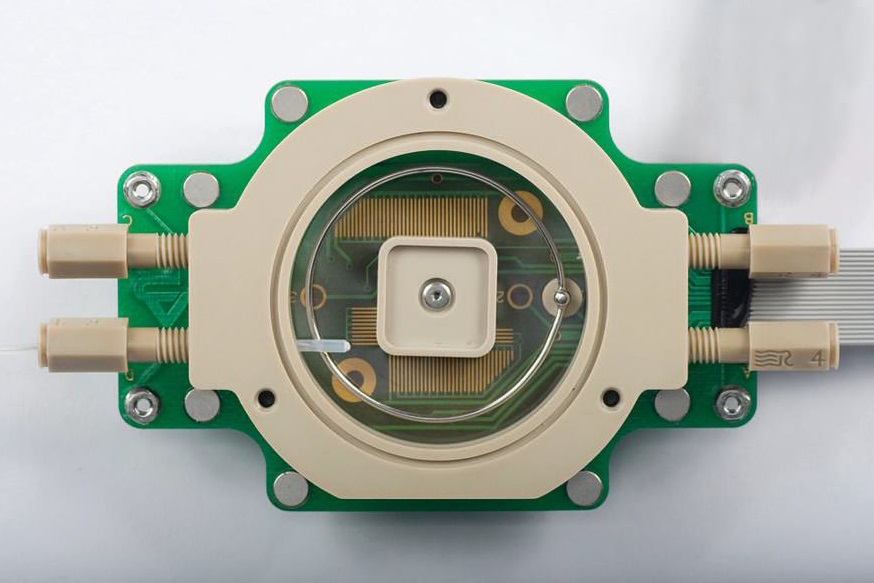 Figure 2. Through its use as an accessory to an atomic force microscope, this electrochemical cell can measure oxidation and corrosion at the nanoscale. (Courtesy of Asylum Research)
Figure 2. Through its use as an accessory to an atomic force microscope, this electrochemical cell can measure oxidation and corrosion at the nanoscale. (Courtesy of Asylum Research)
The electrochemical cell accommodates samples (working electrodes) of various sizes, including metal cylinders, flat conducting samples and even conducting thin films on insulating substances. The cell is supplied with a boron-doped-diamond working electrode, a fully sealed, liquid-filled, silver-silver chloride reference electrode and a carbon counter electrode. Several sealed ports allow for other reference and counter electrodes.
A sample being evaluated can be immersed in a liquid in a closed system that is surrounded by air. Experiments can be done to show how the surface is affected by the liquid. For example, the U.K. group did a study showing the electrochemical deposition of bismuth on the working electrode over time (
2).
The typical sample is one centimeter in diameter and a few millimeters thick. Rutgers says, “Sample weight is not an issue, as the AFM is capable of handling sample weights up to three kilograms.”
The topography of a surface is evaluated by positioning the AFM tip over the top of the sample and scanning in a raster pattern. Deflection of the cantilever due to surface topography is recorded, producing a topographical map of the surface.
Metal wires of up to 1/16 of an inch in diameter can be moved into the cell through two side access ports. Rutgers says, “We have minimized the number of clips and wires by using a junction box. The cell’s wiring connects to the box via flat cable and offers standard connections for plugging in any potentiostat (necessary equipment for driving electrochemical reactions).”
An optional heating element also can be used to evaluate the thermodynamics and kinetics of electrochemical reactions up to temperatures of 90 C. this enables the electrochemical cell to measure such parameters as pKa and acceleration of the corrosion.
Further information on the electrochemical cell can be found at
www.asylumresearch.com or by contacting Monteith Heaton at
monte@asylumresearch.com.
REFERENCES
1.
Canter, N. (2010), “Size Does Matter for Nanoscale Friction,” TLT,
66 (8), pp. 10–11.
2.
Toghill, K., Wildgoose, G., Moshar, A., Mulcahy, C. and Compton, R. (2008), “The Fabrication and Characterization of a Bismuth Nanoparticle Modified Boron Doped Diamond Electrode and its Application to the Simultaneous Determination of Cadmium (II) and Lead (II),”
Electroanalysis,
20 (16), pp. 1731–1737.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.