The Influence of Amine Structure on Performance in MWFs
Patrick E. Brutto | TLT Technical Analysis March 2011
Certain amines can enhance the performance of antimicrobials and could be inhibitory themselves.
www.canstockphoto.com
KEY CONCEPTS
•
MWFs containing components should theoretically last longer and require less maintenance.
•
Several factors can influence the performance of two common biocides: triazine and BIT.
•
When all microbiological and corrosion data are considered, the aromatic amine AR-9-1 performs best with BIT.
Amines and, in particular, amino alcohols (also called alkanolamines) have been used for many years in water-dilutable metalworking fluids. Their primary functions are (1.) neutralization of acid-functional components and (2.) development and maintenance of alkaline pH. Amine salt/soap reaction products function as emulsifi ers, corrosion inhibitors and lubricity agents. In some cases, the unprotonated amines such as triethanolamine provide ferrous metal corrosion control, and some amines such as monoethanolamine are considered bioresistant (
1). This latter property of certain amines or their salt/soap reaction products has become more important in recent years due to the desire for longer-lasting MWFs.
MWFs containing components which resist microbiological attack should theoretically last longer and require less maintenance. Additionally, there are several studies indicating that certain amines can enhance the performance of registered antimicrobials and/or may be inhibitory themselves (
2-6).
What is lacking in the MWF literature is a study of the performance of a wide variety of amine structures of varying carbon number, molecular arrangement and hydroxyl groups, in terms of both microbiological effects with biocides, as well as corrosion control before and during exposure to microorganisms. This article attempts to address this deficiency.
AMINE COMPOUNDS
The amine compounds are described in Figure 1. The “designation” descriptions are as follows: the first two letters indicate molecular structure (AL for aliphatic, CY for cycloaliphatic and AR for aromatic), the middle numbers are the number of carbons and the last number is the number of hydroxyl groups. For example, AL-9-1 is an aliphatic 9-carbon amine with 1-hydroxyl group. As indicated, some of the structures are commercially available, while others are experimental and proprietary.
 Figure 1. Amine Compounds
BIOCIDE COMPOUNDS
Figure 1. Amine Compounds
BIOCIDE COMPOUNDS
The following registered biocides were evaluated in this study:
•
Hexahydro-1,3,5-tris(2-hydroxethyl)-s-triazine, supplied as 78% active ingredient in water. This biocide will be referred to as “triazine.”
•
1,2-Benzisothiazolin-3-one, supplied as 20% active in water/dipropylene glycol. This biocide will be referred to as “BIT.”
MWF FORMULATION
The following low-oil, boron-free semisynthetic MWF formulation was selected for this study (
see Figure 2).
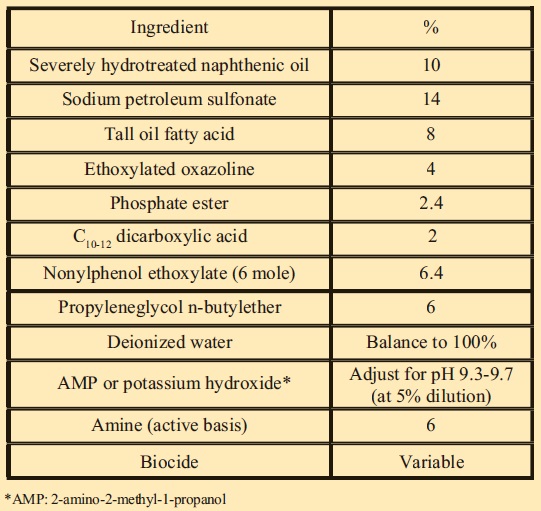 Figure 2. MWF Formulation
MICROBIOLOGY TEST PROTOCOL
Figure 2. MWF Formulation
MICROBIOLOGY TEST PROTOCOL
Tests were conducted in accordance with ASTM Practice E2275. MWF samples containing each amine/biocide combination were diluted to 5% by weight using Lake Michigan tap water (125 ppm total hardness, chlorinated/fluorinated). A mixed bacterial/fungal inoculum was isolated from field samples of used MWFs. This inoculum was added to 400 mL of diluted MWF in a 500-mL Erlenmeyer flask at a dosage of 1x10
6 colony forming units (CFU/mL) bacteria, and 1x10
4 CFU/mL fungi. A small amount of standard grey cast iron chips was added to the flask, which was then loosely sealed with a polyether foam plug (
7). The flask was mixed continuously on an orbital shaker for five days and then allowed to set undisturbed over the weekend.
This cycling was continued until bacterial and fungal growth exceeded the failure point, indicated by two consecutive weeks at 1x10
5 CFU/mL bacteria or 1x10
3 CFU/mL fungi. Tap water was added as needed to maintain the original fluid volume. The microbial counts were measured weekly using a standard serial dilution/plate count method as described in ASTM Method D5465.
CAST IRON-CHIP CORROSION TEST PROTOCOL
Corrosion of grey cast iron chips in contact with the freshly diluted and microbially-aged MWFs was evaluated using a modified ASTM D4627 procedure. Exactly 3.0 grams of chips were weighed into a small plastic petri dish containing a white filter paper. The chips were covered completely with 5.0 grams of test fluid. The dish was covered with a plastic lid and held at room temperature for two hours. The fluid was removed using a pipet, and the chips dried for 24 hours (on the filter paper in the open petri dish) at 25 °C and 60% relative humidity. The chips were removed from the filter paper and the percent stain estimated visually.
MICROBIOLOGY AND CORROSION RESULTS WITH TRIAZINE
Results of microbiological testing of fluids with triazine biocide (550 ppm active at dilution) are presented in Figures 3 and 4. These charts present the weeks of bacterial and fungal control as a function of the amine in the formulation (3,000 ppm active at dilution). Figure 3 presents the results with the aliphatic amines. Bacterial control with triazine is better with certain C6-12 amines present. One exception is AL-8-2 where the presence of two hydroxyl groups reduces performance relative to a similar compound with one hydroxyl, AL-8-1.
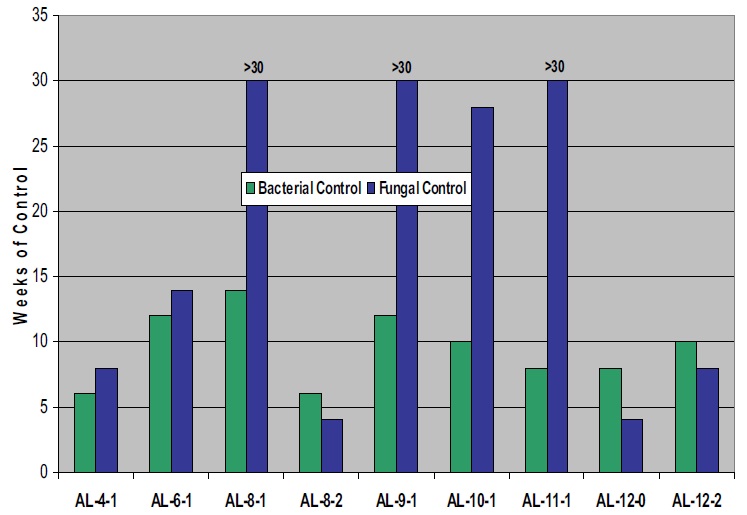 Figure 3. Microbial Control with Triazine and Aliphatic Amines
Figure 3. Microbial Control with Triazine and Aliphatic Amines
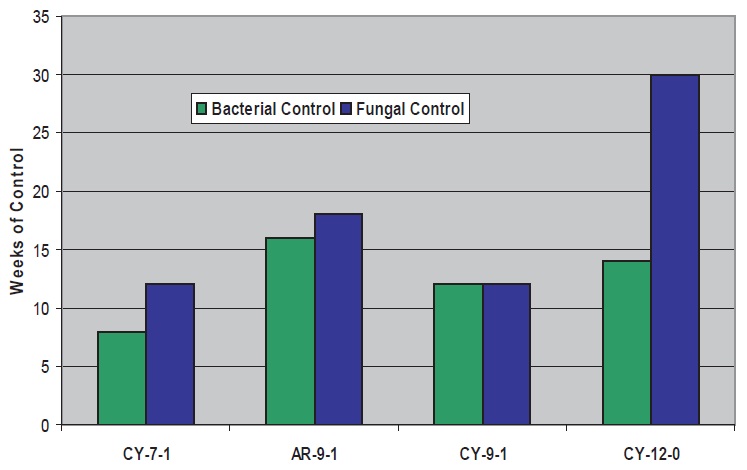 Figure 4. Microbial Control with Triazine and Cycloaliphatic/Aromatic Amines
Figure 4. Microbial Control with Triazine and Cycloaliphatic/Aromatic Amines
The differences in fungal control as a function of amine are much greater, with several of the C
8-11 compounds performing significantly better than the others. The performance of AL-8-2 is again inferior to AL-8-1, indicating that the number of hydroxyl groups, in addition to carbon number, is important.
For the cycloaliphatic and aromatic amines (
see Figure 4), bacterial control with triazine is in the same range as with the aliphatics, with C
9-12 performing better than C
7. However, efficacy against fungi is significantly less with the C
9 cycloaliphatic and aromatic amines, and significantly greater with the C
12 cycloaliphatic amine (relative to aliphatics with the same number of carbons/hydroxyls). The aromatic C
9 amine performs better than the cycloaliphatic C
9.
Cast iron corrosion control data are presented in Figures 5 and 6 for the fresh and microbially-aged fluids. Data for the aliphatic amines (
see Figure 5) show the best performance with several C
8-12 amines where initial corrosion control is superior and control is maintained during microbial aging. Exceptions are noted for amines with two hydroxyls (AL-8-2 & AL-12-2) where performance is inferior relative to amines with the same carbon number but fewer hydroxyls. This appears to be due to a combination of poorer inherent corrosion control properties and poorer microbiological control.
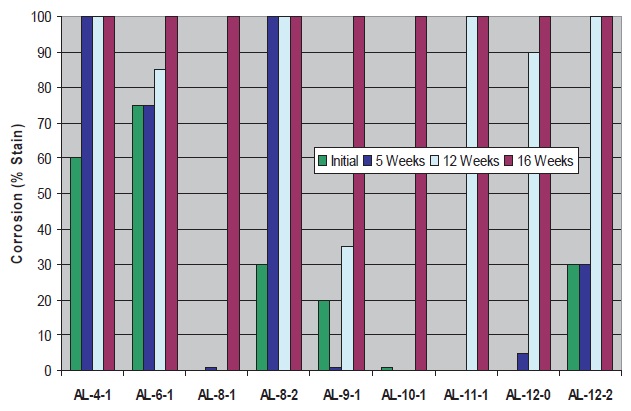 Figure 5. Cast Iron Corrosion Control of Fluids with Triazine and Aliphatic Amines
Figure 5. Cast Iron Corrosion Control of Fluids with Triazine and Aliphatic Amines
With the cycloaliphatic amines (
see Figure 6), cast iron corrosion control is similar to aliphatics with the same number of carbons/hydroxyls. The aromatic C
9 amine is less effective than its aliphatic/cycloaliphatic analogues, however, this amine provides the best corrosion control at the longest microbial aging time. This could be related to the superior bacterial control in this fluid at the 15-16 week period.
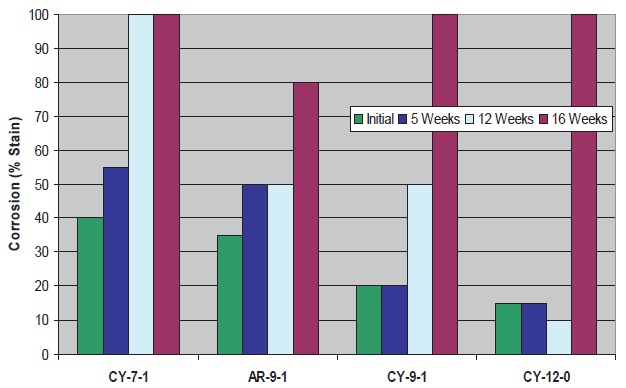 Figure 6. Cast Iron Corrosion Control of Fluids with Triazine and Cycloaliphatic/Aromatic Amines
Figure 6. Cast Iron Corrosion Control of Fluids with Triazine and Cycloaliphatic/Aromatic Amines
When all microbiological and corrosion data are considered, the aliphatic amine AL-8-1 performs best overall with triazine biocide.
MICROBIOLOGY AND CORROSION RESULTS WITH BIT
BIT is normally ineffective in controlling bacterial or fungal growth in MWFs. However, because this biocide is not formaldehyde-based and is stable at alkaline pHs, MWF producers would like to use it where formaldehyde-based products are undesirable or not allowed due to regulation.
Microbiological control results for fluids containing 180 ppm BIT and 3,000 ppm amine (active basis at 5% dilution) are presented in Figures 7 and 8. The ineffectiveness of BIT is demonstrated in the fluid with amine AL-4-1 (
see Figure 7); this amine is acknowledged in the industry as one of the more bioresistant products available. Significant improvements in BIT performance are seen when several of the C
8-12 aliphatic amines are used. AL-9-1 performs particularly well with BIT. Performance deficiencies are again noted with amines having two hydroxyl groups (AL-8-2 and AL-12-2) vs. those with 0-1 hydroxyls.
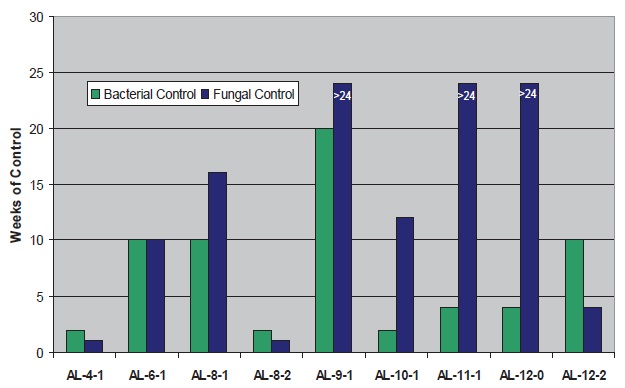 Figure 7. Microbial Control with BIT and Aliphatic Amines
Figure 7. Microbial Control with BIT and Aliphatic Amines
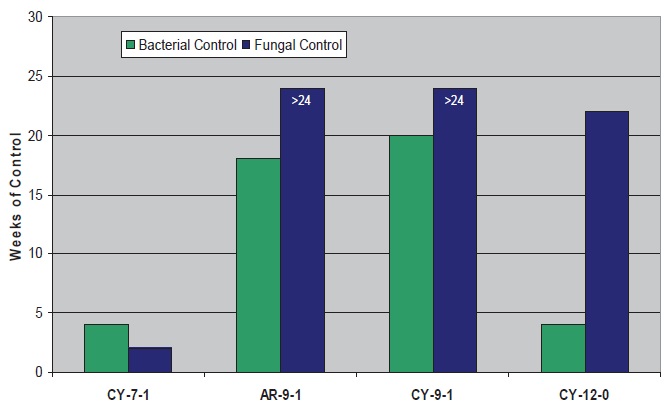 Figure 8. Microbial control with BIT and cycloaliphatic/Aromatic Amines
Figure 8. Microbial control with BIT and cycloaliphatic/Aromatic Amines
For the cycloaliphatic and aromatic amines (
see Figure 8), the C
9 molecules are best with BIT and perform similarly to the best aliphatic, also a C
9 (AL-9-1).
Cast iron corrosion control data are presented in Figures 9 and 10. Performance is best for fluids with certain C
8-12 aliphatic amines, and generally decreases when two hydroxyl groups are present (vs. 0-1). The results indicate amines in this range provide inherently better corrosion control, and maintenance of corrosion control correlates well with microbial control.
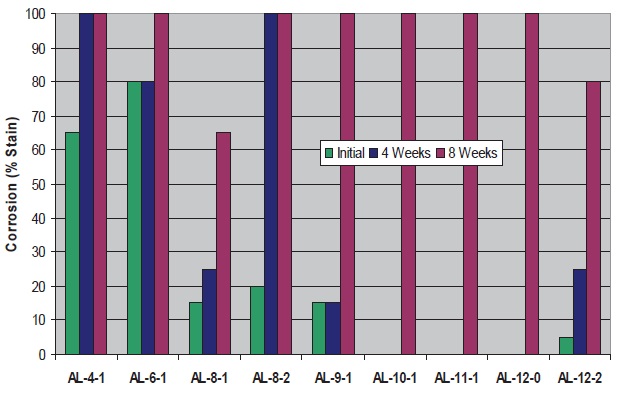 Figure 9. Cast Iron Corrosion Control of Fluids with BIT and Aliphatic Amines
Figure 9. Cast Iron Corrosion Control of Fluids with BIT and Aliphatic Amines
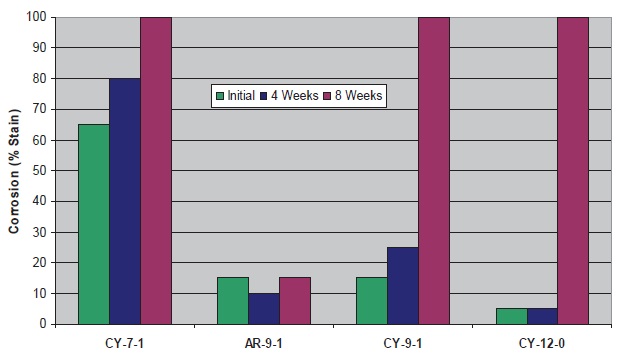 Figure 10. Cast Iron Corrosion Control of Fluids with BIT and Cycloaliphatic/Aromatic Amines
Figure 10. Cast Iron Corrosion Control of Fluids with BIT and Cycloaliphatic/Aromatic Amines
For the cycloaliphatic and aromatic amines, performance is best at C
9-12. The advantage of the C
9 aromatic (vs. C
9 cycloaliphatic) was not expected based on the microbial control results. This could indicate less biodegradation of the aromatic, but other explanations are also possible.
When all microbiological and corrosion data are considered, the aromatic amine AR-9-1 performs best with BIT.
CONCLUSIONS
Amine molecular size, structural configuration and number of hydroxyl groups have been shown to influence the performance of two common biocides: triazine and BIT. These factors also influence the inherent corrosion control provided by these amines, as well as maintenance of corrosion control.
Amines in the C
8-12 range with 0-1 hydroxyl groups enhance biocide performance more than those outside this carbon range and/or with 2-hydroxyl groups. C
8-12 amines also provide the best cast iron corrosion control. The best overall performance with triazine is with a C
8 aliphatic amine, and with benzisothiazolinone a C
9 aromatic amine performs best.
ACKNOWLEDGMENTS
The author would like to acknowledge Chuck Coburn, David Green, Arthur Jones, C.J. Nash, John Pohlman, Bonnie Pyzowski and Ray Swedo with ANGUS Chemical Co., a subsidiary of The Dow Chemical Co. These individuals were responsible for synthesis of the experimental amine compounds and all the test results presented in this article.
REFERENCES
1.
Byers, J.P. (2006),
Metalworking Fluids, Second Edition, CRC Press, pp. 132, 138-141, 144.
2.
Bennett, E.O. (1979), “Antimicrobial Properties of Butanolamines and Propanolamines in Metalworking Fluids,”
J. Gen. Appl. Microbiol.,
25, pp. 63-69.
3.
Bennett, E.O. (1979), “Corrosion Inhibitors as Preservatives for Metalworking Fluids Ethanolamines,”
Lubrication Engineering,
35 (3), pp. 137-144.
4.
Oppong, D. (1989), “Use of a Mixture of Alcoholamines to Potentiate the Antimicrobial Activities of Certain Cutting Fluid Preservatives,”
Tribology International,
22-5, pp. 343-346.
5.
Sandin, M. (1992), “The Role of Alkyl Chain Length on the Antibacterial Activity Of Alkyl Ethanolamines,”
Biomedical Letters,
47, pp. 85-92.
6.
Rossmoore, H.W. (1993), “Biostatic Fluids, Friendly Bacteria and Other Myths in Metalworking Microbiology,”
Lubrication Engineering,
49 (4), pp. 253-268.
7.
Plugs Supplied By Jaece Industries, Inc., North Tonawanda, N. Y., Part number L800-C.
 Patrick Brutto is Development Leader-Dow Performance Materials, based in Buffalo Grove, Ill. You can reach him at pebrutto@dow.com
Patrick Brutto is Development Leader-Dow Performance Materials, based in Buffalo Grove, Ill. You can reach him at pebrutto@dow.com.