New lithium-ion battery technology
Dr. Neil Canter, Contributing Editor | TLT Tech Beat March 2011
Researchers develop a nanomaterial for high-power output applications.
KEY CONCEPTS
•
Silicon technologies used as anodes in lithium-ion batteries are either not durable or cannot generate sufficient power output for use in automobile applications.
•
A new anode material has been developed in the shape of an ice cream cone and is known as a nanoscoop.
•
The nanoscoop is able to handle the volumetric strain from continuous charge/discharge cycles and also display a higher power output.
The rapid movement of the automotive industry to develop electric vehicles such as Gm’s Chevy Volt has accelerated the push to find a battery technology that provides excellent cost performance. As noted previously in this column, lithium-ion batteries show great promise as an energy source but have encountered problems such as slow cycling (charge and discharge) rates of lithium ions and the potential to catch fire during use.
Research on new lithium-ion batteries has moved to the preparation of nanoscale materials. In a previous TLT article, development of two-dimensional, network-Web heteronanostructures described as nanonets based on silicon-coated, titanium disilicide was described (
1). The nanonet can be used as an anode material in a silicon battery and shows a superior charge/recharge rate compared to a typical anode material.
Nikhil Koratkar, professor in the mechanical, aerospace and nuclear engineering department at Rensselaer Polytechnic Institute in Troy, N.Y., provides a perspective on the types of anode materials evaluated in lithium-ion batteries. He says, “The first material looked at was graphite, which is mechanically strong and stable. But the species formed with lithium (lithium hexacarbide) did not allow for sufficient lithium to be stored in the graphite.”
Attention then turned to silicon because the main species (Li
22Si
5) contains one silicon atom for every 4.4 lithium atoms. Koratkar explains that silicon also creates problems because the cycling of lithium atoms into and then out of this material can lead to rapid changes in expansion and then contraction. He says, “Silicon is very brittle and within a few tens of cycles will readily break apart under the stress of volumetric expansions.”
Koratkar indicates that crystalline silicon can expand up to 400% during the charging process while amorphous silicon can expand up to 280%. This problem led researchers to examine silicon nanowires, nanotubes and nanoparticles.
Koratkar says, “Silicon nanostructures handle the stress of volumetric expansion much better, but in all of the work reported they generate only a low-power output.” This type of battery will work well for many applications that do not need high output such as a sensor.
But they are not adequate for use in high-power output applications such as an automobile where a lot of power must be drawn to accelerate. A new approach has now been developed for an anode material that has the potential to be used in this type of application.
NANOSCOOPS
One of the problems with nanostructures is their incompatibility with the main conducting substrate used, which is stainless steel or copper. Koratkar notes that the interface between the substrate and silicon nanostructures is vulnerable to cracking during high-rate charge/recharge cycling.
Koratkar and his fellow researchers have developed a new nanomaterial that shows much greater promise for maintaining a high-power density and a high-energy density over a long operating period. He says, “We decided to prepare an anode material that contains carbon, amorphous silicon and aluminum in order to better withstand the strain occurring during the uptake and discharge of lithium ions.”
The carbon is most compatible with the stainless steel substrate but shows a much smaller volumetric strain (approximately 10%) as compared to amorphous silicon. Aluminum is utilized between them because it expands and contracts at a percentage (94%) between carbon and silicon.
Koratkar says, “This type of structure works for two main reasons. First, the strain gradient in moving from carbon to silicon is much better able to handle the stress of the volumetric expansion. Second, the nanoscale size of this anode material means there is not a lot of time taken for the lithium ions to soak into this structure.”
A scanning electron microscope image of the anode material is shown in Figure 2. The silicon layer is outlined in purple, the aluminum in green and the carbon in pink. The carbon nanorods are approximately 170 nanometers long, the aluminum layer 10 nanometers long and the silicon scoop is approximately 60 nanometers thick.
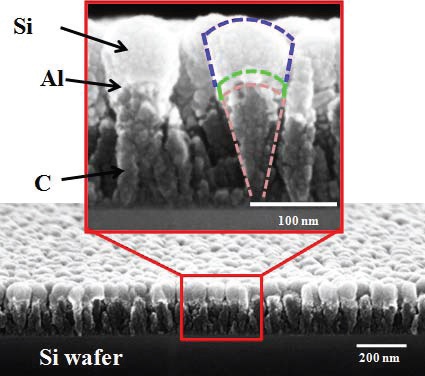 Figure 2. A scanning electron microscope image of a new lithium-ion battery anode material shows a gradient of silicon, aluminum and carbon that has the appearance of a scoop of ice cream. This nanoscoop displays better durability and generates a high-power output making it useful for applications such as automobiles. (Courtesy of Rensselaer Polytechnic Institute)
Figure 2. A scanning electron microscope image of a new lithium-ion battery anode material shows a gradient of silicon, aluminum and carbon that has the appearance of a scoop of ice cream. This nanoscoop displays better durability and generates a high-power output making it useful for applications such as automobiles. (Courtesy of Rensselaer Polytechnic Institute)
This material, as viewed at the nanoscale, has the appearance of an ice cream cone with a scoop of ice cream on top. For this reason, the researchers designated the anode material as a nanoscoop.
The researchers demonstrated that the nanoscoops can handle high-frequency charge/discharge rates. Koratkar says, “We found that the nanoscoops functioned very well at a charge/discharge rate of 40 C, which represents one cycle occurring every 90 seconds. The nanoscoops also displayed good durability over 100 continuous charge/discharge cycles.”
The power densities obtained from the electrode at the 40 C charge/discharge rate was one order of magnitude better than with a conventional battery anode. When attempts were made to increase the rate to 100 C (one cycle every 35 seconds), no volume change was observed, which means that lithium ions did not diffuse into the silicon layer but, rather, engaged in a surface reaction.
Koratkar indicates that even at a rate of 40 C, the lithium ions only diffuse into about a third of the silicon region, so there is room for improvement. In spite of this partial lithiation, the energy density (or charge storage density) of the electrode was comparable to conventional battery anodes.
Future work will look at seeing how to scale the nanoscoop design so that it could conceivably be used to power an automobile. Koratkar says, “We are looking to stack nanoscoops on top of each other to see if they can work together to generate higher power levels. Currently, we are evaluating nanoscoops with three layers vs. two layers vs. one layer. A second approach involves placing the nanoscoops via roll-to-roll deposition on a large area flexible sheet that can be rolled into a more compact shape so that it can be installed in an automobile.”
A final approach is to use thermal evaporation instead of sputtering to prepare the nanoscoops. This technique enables the preparation of nanostructured materials with increased length or height. Koratkar says, “We have prepared nanoscoops that are 20 microns long, which is two orders of magnitude longer than the currently prepared materials. Evaluation of these longer nanoscoops is pending.
Further information can be obtained in a recent publication (
2) or by contacting Koratkar at
koratn@rpi.edu.
REFERENCES
1.
Canter, N. (2010), “Faster, More Durable Lithium-Ion Batteries,” TLT,
66 (6), pp. 14–15.
2.
Krishnan, R., Lu, T. and Koratkar, N. (2010), “Functionally Strain-Graded Nanoscoops for High Power Li-Ion Battery Anodes,”
Nano Letters, DOI: 10.1021/nl102981d.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.