Stable fuel cell catalyst
Dr. Neil Canter, Contributing Editor | TLT Tech Beat February 2011
Researchers develop a new catalyst that exhibits better stability and performance.
KEY CONCEPTS
•
The main catalysts used in fuel cells are not durable.
•
A new catalyst that contains a monolayer of platinum on palladium nanoparticles provides enhanced stability.
•
The slightly higher reactivity of palladium enables the platinum to become more stable over time.
Development of fuel cells is continuing because this technology has the potential to generate sufficient electricity to power automobiles in an environmentally friendly manner. Fuel cells facilitate the oxidation of hydrogen at the anode and reduction of oxygen at the cathode to produce current (flow of electrons between them) with water as the reaction byproduct.
The most promising fuel cell type contains a polymer electrolyte membrane that uses platinum nanocatalysts at the anode and cathode to accelerate the electricity- producing reaction of hydrogen and oxygen. The most common membrane is derived from perfluorinated polymers. A previous TLT article described the development of a hydrocarbon-based polymer that was claimed to exhibit superior performance (
1).
The main catalysts used are based on platinum due to superior performance. But these catalysts do not retain their activity over time. Dr. Radoslav Adzic, chemist at the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory in Upton, N.Y., says, “The durability of platinum fuel cell catalysts is unsatisfactory. In studies conducted over the last three years to assess the stop-and-go driving of electric cars, commercial platinum catalysts on a porous carbon support exhibited a gradual decline in performance to the point where their activity was unsatisfactory after approximately 50,000 potential cycles.”
Platinum nanoparticles dissolve in the membrane, and platinum ions can be reduced by hydrogen molecules diffusing from the anode. This step leads to a rapid loss of catalytic activity.
Adzic indicates that the objective of the study was to have the catalyst perform for 200,000 cycles, which simulates about 10 years of driving. The challenge in using platinum is its availability and price. Adzic adds, “Platinum is not readily available and is expensive. A catalyst needs to be developed to extend the performance of platinum while limiting its use.”
Such a catalyst has now been developed.
PLATINUM MONOLAYER
Adzic and his fellow researchers have prepared a new catalyst that contains a monolayer of platinum on palladium nanoparticles maintained on a porous carbon support. This catalyst exhibits much better stability and performance over a larger number of cycles.
Adzic says, “We found that in testing over 100,000 cycles, the platinum monolayer, palladium catalyst only lost 37% of its reactivity, which is a substantial upgrade over conventional fuel cell catalysts. The reason for the enhanced stability is that the palladium being more susceptible to dissolution than platinum undergoes dissolution first and, thus, protects platinum.”
A high-angle-annular dark field image of the platinum monolayer on the palladium is shown in Figure 2. Adzic explains, “Platinum has about twice the number of electrons as palladium, so the monolayer is slightly brighter than the palladium using this technique.”
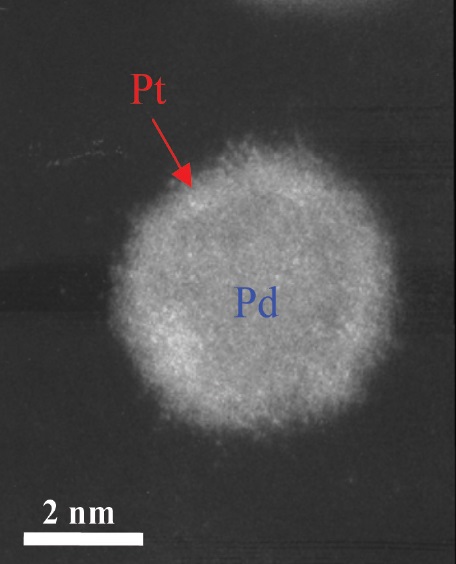 Figure 2. A more stable fuel cell catalyst contains a monolayer of platinum, as shown in this high-angle annular dark field image. (Courtesy of the U.S. Department of Energy’s Brookhaven National Laboratory)
Figure 2. A more stable fuel cell catalyst contains a monolayer of platinum, as shown in this high-angle annular dark field image. (Courtesy of the U.S. Department of Energy’s Brookhaven National Laboratory)
The new fuel cell catalyst was prepared through a copper intermediate. A technique known as underpotential deposition was used to place a copper monolayer on reduced surfaces of palladium nanoparticles. Galvanic displacement of copper monolayer by platinum monolayer was achieved through immersion of the palladium nanoparticles covered by a copper monolayer in a platinum solution, according to Adzic.
Evaluation of the platinum monolayer catalyst was conducted using a real 25-square centimeter fuel cell prepared by Brookhaven’s partner, Toyota Motor Corp. Adzic says, “A potential program was applied, according to doe protocol. Polarization characteristics were measured from time to time. At the end of the testing, the fuel cell was dismantled and a cross section examined to determine how much palladium dissolved and whether it formed a band in the middle of the cell.”
Analysis of the cross section showed that a significant amount of palladium was oxidized, dissolved and moved away from the cathode. The palladium ions were reduced by hydrogen diffusing from the anode to form a band, but the platinum monolayer was not affected.
Adzic believes that the stability of the platinum monolayer catalyst is due to the slightly higher reactivity of palladium. He says, “Palladium acts in a similar fashion to a sacrificial anode by dissolving first. Palladium has a lower standard electrochemical potential than platinum and, as such, gets oxidized before platinum. The resulting palladium ions then diffuse through imperfections in the platinum monolayer.”
This loss of palladium leads to a contraction of the shell, which makes the platinum monolayer catalyst more stable and less prone to ion dissolution.
Gold is another metal that can be used in this catalyst. By alloying gold and palladium, the stability of palladium and of the whole catalyst increases. The researchers developed a fuel cell catalyst with 10% gold and found a loss of only 30% reactivity after 200,000 cycles of testing.
When asked if this platinum monolayer concept could be used to improve the durability of an automotive emissions catalyst, Adzic is uncertain. He says, “We have not done any work on emissions. A platinum monolayer catalyst can be tried, but it cannot be prepared on a porous carbon support.”
The platinum monolayer catalyst currently exhibits performance that exceeds the goal set by doe for research conducted between 2010 and 2015. Adzic is confident that this catalyst can be used in future automotive applications. He says, “We will continue doing our work in conjunction with our partners to further improve the durability of this catalyst. The fact that it uses a much smaller amount of platinum enhances the commercial viability of this catalyst.”
Further information can be obtained from a recent article (
2) or by contacting Adzic at
adzic@bnl.gov.
REFERENCES
1.
Canter, N. (2005), “More Efficient Fuel Cell Membrane,” TLT,
61 (5), pp. 10–12.
2.
Sasaki, K., Naohara, H., Cai, Y., Choi, Y., Liu, P., Vukmirovic, M., Wang, J. and Adzic, R. (2010), “Core-Protected Platinum Monolayer Shell High-Stability Electrocatalysts for Fuel-Cell Cathodes,”
Angewandte Chemie International Edition,
49 (46), pp. 8602–8607.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.