Chloride binder
Dr. Neil Canter, Contributing Editor | TLT Tech Beat January 2011
A newly developed reversible binder could minimize corrosion in lubricant systems.
KEY CONCEPTS
•
There are not many ways to prevent chloride anions from corroding steel alloys.
•
A reversible binder has been developed that has peak selectivity for chloride anions.
•
The new binder more effectively binds chloride anions in the presence of visible light and releases chloride anions in the presence of ultraviolet light.
Corrosion has been a major topic in this column because it causes significant problems and leads to premature machinery failure in a wide range of lubricant applications. Research is actively progressing to develop better corrosion inhibitors that are more effective in minimizing the spread of corrosion.
In a recent TLT article, a naturally derived corrosion inhibitor produced by a specific bacteria strain was described (
1). Bacteria normally produces byproducts that are acidic in nature and can lead to corrosion. But a specific strain of bacteria produces an exopolysaccharide coating that has been found to inhibit corrosion of carbon steel.
In dealing with corrosion, few answers have been found for how to prevent chloride from attacking steel alloys. Chloride is an anion that is prevalent in our environment. When thinking about the presence of chloride, seawater comes to mind.
One approach to eliminate chloride anions from a system is the use of a specific agent that can literally remove it chemically through the use of a receptor. Dr. Amar Flood, assistant professor of chemistry at Indiana University in Bloomington, Ind., says, “There are a lot of different types of organic chelators that exhibit some degree of anionic binding. The strength and selectivity of the chelator one needs depends on the boundary conditions of the application.”
A chelating agent that is specially prepared to bind chloride could potentially be used in a lubricant system to minimize the potential for corrosion. Such a technology has not been available until now.
SWITCHABLE FOLDAMER
Flood and his researchers developed a binder that has peak selectivity for chloride anions. The binder is an aryl-triazole foldamer containing two azobenzene end-groups.
Flood says, “We found that a favorable orientation of this foldamer will preferentially bind chloride anions. A change in conditions will prompt the foldamer to change its shape and release the chloride anion. Once the chloride anion is released, the original operating conditions can be re-established to enable the foldamer to bind a second chloride anion. This switchable behavior can enable the user to bind chloride anions and then release them at another location where they can be isolated and removed from a system.”
The behavior of the foldamer is similar to how a protein changes its conformation. Flood says, “A foldamer is a compound present in one specific conformation. This is similar to a protein that can exist either in an alpha helix or beta sheet orientation.”
In the case of the aryl-triazole derivative, this foldamer is switchable because it can unfold from a specific conformation to a random coil, as shown in Figure 2. The chloride atom (shown in green) is held within the folded conformation in the compound on the left and then is released when the random coil geometry is present on the right.
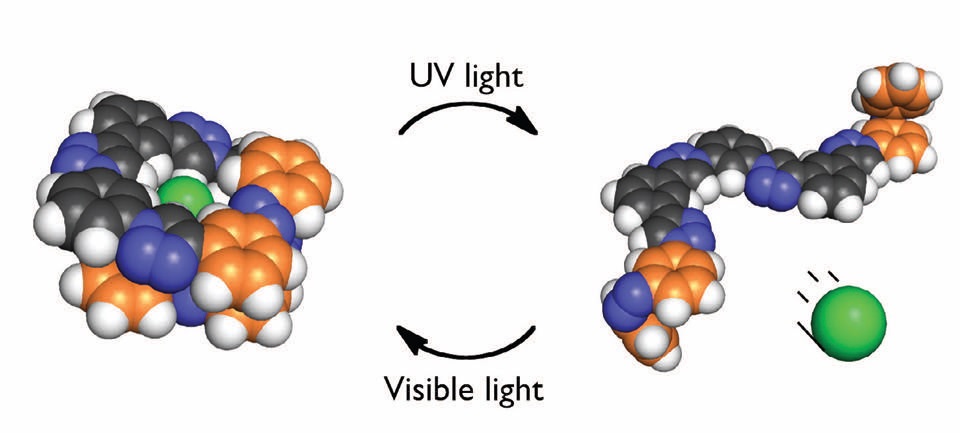 Figure 2. The foldamer assumes a favorable trans-trans orientation which effectively binds a chloride anion (shown in green) in the presence of visible light. When ultraviolet light is applied, the chloride anion is released as the foldamer is converted to a cis-cis isomer. (Courtesy of Indiana University)
Figure 2. The foldamer assumes a favorable trans-trans orientation which effectively binds a chloride anion (shown in green) in the presence of visible light. When ultraviolet light is applied, the chloride anion is released as the foldamer is converted to a cis-cis isomer. (Courtesy of Indiana University)
Flood indicates that the 1,2,3-trazole functionality is particularly good for binding chloride. He says, “This functionality withdraws electrons that help to heavily polarize the adjacent carbon-hydrogen bond so that it can participate in hydrogen bonding. The result is the hydrogen is available to bind with the chloride anion.” In the case of the aryl-triazole foldamer, the more thermodynamically stable conformation is a trans-trans isomer that readily binds chloride anions. The random coil is a less stable cis-cis isomer that releases the chloride anion.
As shown in Figure 2, ultraviolet light photoisomerizes the trans-trans isomer to the cis-cis isomer. The process can be reversed in the presence of visible light.
Flood says, “The UV light reaction converts the transtrans isomer to a mixture that is 2/3 cis-cis and 1/3 cis-trans. If the mixture is left for one day at room temperature, it reverts back to the trans-trans isomer, even in the absence of light.”
The researchers titrated the aryl-triazole foldamer with the chloride source, tetrabutylammonium chloride in acetonitrile, to evaluate its binding capability. Titrations were run in the presence of both visible and ultraviolet light.
Flood says, “We found that the binding capability of the aryl-triazole foldamer was 10 times higher with the transtrans isomer than the cis-cis isomer. To examine how this foldamer can control chloride concentrations, we measured the conductivity of a salt solution. Changing the light source can change the conductivity. Conductivity declined in the presence of visible light, which means that the trans-trans isomer is binding more chloride anions. A change to ultraviolet light led to an increase in conductivity reflective of the decrease in the binding of the chloride anion as the aryltriazole foldamer is transformed to the cis-cis isomer.”
The researchers have evaluated the ability of the aryltriazole foldamer to bind chloride through 10 to 20 cycles. Flood reports that the binder retains its affinity for chloride anions through these cycles.
The aryl-triazole foldamer can be prepared by the cycloaddition of an alkyne with an azide in the presence of a copper-(i) catalyst. Flood says, “This is a very versatile process that is known as click chemistry.”
The current work showing the performance of the chloride binder was done in an organic solvent. Flood says, “We know that development of a binder compatible with water is a key application for this technology. An aqueous environment presents its own challenges because water readily solvates chloride anions. This effect will need to be overcome.”
Future work will focus on using click chemistry to develop a switchable foldamer that can be used in water. Flood is also looking to improve the difference in binding between the stable trans-trans conformation and the less stable cis-cis transformation. He adds, “Currently we see a 10-fold difference in binding between the isomers. Our goal is to improve the difference to 1,000-fold.”
Further information can be found in a recent article (
2) or by contacting Flood at
aflood@indiana.edu.
REFERENCES
1.
Canter, N. (2010), “Natural Corrosion Inhibitor from Bacteria,” TLT,
66 (11), pp. 8–9.
2.
Hua, Y. and Flood, A. (2010), “Flipping the Switch on Chloride Concentrations with a Light-Active Foldamer,”
J. Am. Chem. Soc.,
132 (37), pp. 12838–12840.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.