Super-strong, ductile aluminum
Dr. Neil Canter, Contributing Editor | TLT Tech Beat January 2011
A new technique has been developed that increases the strength of aluminum to that of carbon steel alloys.
KEY CONCEPTS
•
Aluminum typically is strengthened through age-hardening, a 100-year-old technique.
•
A new process, high-pressure torsion, increases the strength of aluminum to a level comparable to carbon steel—without sacrificing ductility.
•
This process leads to the formation of smaller grains below 100 nanometers in diameter and hierarchical nanosize clusters that appear to strengthen the aluminum.
The need for greater efficiency and productivity in our automobiles has lead to research in developing lighter metal alloys with comparable strength to conventional steel. Use of these metals should lead to a reduction in the weight of the automobile, which translates into greater fuel economy.
In a previous TLT article, we described research on a relatively new type of material known as metallic glasses (
1). metallic glasses are prepared from zirconium and display diameters less than 100 nanometers. Testing showed they displayed high levels of mechanical strength combined with ductility.
Aluminum has been an attractive metal for use in automobiles because of its lighter weight compared to steel, good strength and ability to elongate. But aluminum has not reached the mechanical strength achieved by steel.
Dr. Yuntian Zhu, professor of materials science at North Carolina State University in Raleigh, N.C., says, “The traditional technique used to strengthen aluminum alloys is age-hardening, which has been known for over 100 years. If pure aluminum is cut open and evaluated under a microscope, a lot of crystalline grains are seen. Within each grain, the atoms are arranged in a regular order. Heat treatment of aluminum produces a change in the solubility of fine particles in the aluminum matrix. This leads to the formation of a different phase of smaller particles within the aluminum matrix that impedes the movement of dislocations.”
This effect reduces the ductility of the aluminum and enhances its mechanical strength or hardness. Small particles inside grains act as barriers to the dislocation movement. Zhu makes an analogy to driving on a highway. “if you are driving along on a highway at 100 miles per hour,” he says, “and the surface is smooth and even, age-hardening is similar to scattering rocks on the highway, which makes moving along the surface at the same rate of speed difficult.”
This means the speed of the automobile needs to be reduced, which, in effect, is a direct indication that the metal’s strength has increased.
Conventional aluminum exhibits maximum yield strength of 0.4 gigapascals (GPa). Age-hardening further increases the strength of aluminum to 0.7 GPa while elongation, a measure of ductility, remains relatively constant. The average particle size in an age-hardened aluminum is greater than 150 nanometers.
Other techniques such as severe plastic deformation have been tried to further increase the strength of aluminum. But there is need for doing further research to see if a technique can be developed to increase the strength of aluminum closer to that of steel. Zhu says, “If we can further reduce the grain size in the aluminum alloy below 100 nanometers, then the strength of the resulting metal should significantly increase.” Such a technique has now become available.
HIGH-PRESSURE TORSION
Zhu and his fellow researchers further treated a well-known and used aluminum aerospace alloy, 7075, in a severe plastic deformation technique known as high-pressure torsion. In this process, a disc-shaped sample is sheared between two anvils that are rotated with respect to each other.
In doing the study, researchers initially solution-treated 7075 aluminum at 480 C for five hours followed by quenching in room-temperature water. This age-hardening process was followed by high-pressure torsion for 10 revolutions under a pressure of 6 GPa at room temperature. Zhu categorizes high-pressure torsion as a nasty technique.
Tensile strength testing on the resulting aluminum alloy (designated as NH-7075) showed that this metal exhibits a strength of 1.0 GPa, comparable to a typically hardened and tempered carbon-steel alloy. Elongation of the aluminum does not decrease, leading to a metal alloy that becomes much stronger without reducing its ductility. This high strength aluminum alloy also shows no evidence of brittleness.
Zhu believes the reason for the dramatic increase in alloy strength is the formation of smaller grains below 100 nanometers in diameter and the formation of hierarchical nanosize clusters of alloy elements. In fact, high-resolution transmission electron microscopy shows that the average grain size for NH-7075 produced in this study is 26 nanometers.
The researchers developed a new atom probe tomography method to better understand the structure of NH-7075. They found that the metal exhibits a nanostructure with a series of clusters, lines and nodes. Zhu says, “The nodes are 3.7 to 4 nanometers in diameter, and the lines are 18 nanometers in length with a diameter of 4 nanometers.”
An analysis was done to determine the concentration of aluminum and the other metals involved in the alloy in these species. The clusters represent just about 38.5% of the total metal and primarily contain four to seven atoms. Aluminum is the main component at just under 36%, but both zinc and magnesium are quite prominent at levels just below 29% each. Zhu says, “The clusters are important because they make it hard to move the dislocations in the alloy, thereby increasing its strength. Yet, application of enough stress still allows the dislocations to move, which does not hinder the ductility of the NH-7075.”
The combined concentration of lines and nodes is below 10% of the total metal with the remainder of the atoms being in a non-clustered solid solution. Figure 1 shows atom probe tomography of various views of the NH-7075. The different colors are reflective of aluminum and the other atoms (copper, silicon, chromium and titanium) used to prepare NH-70-75. The scale width of the images in this figure is 10 nanometers.
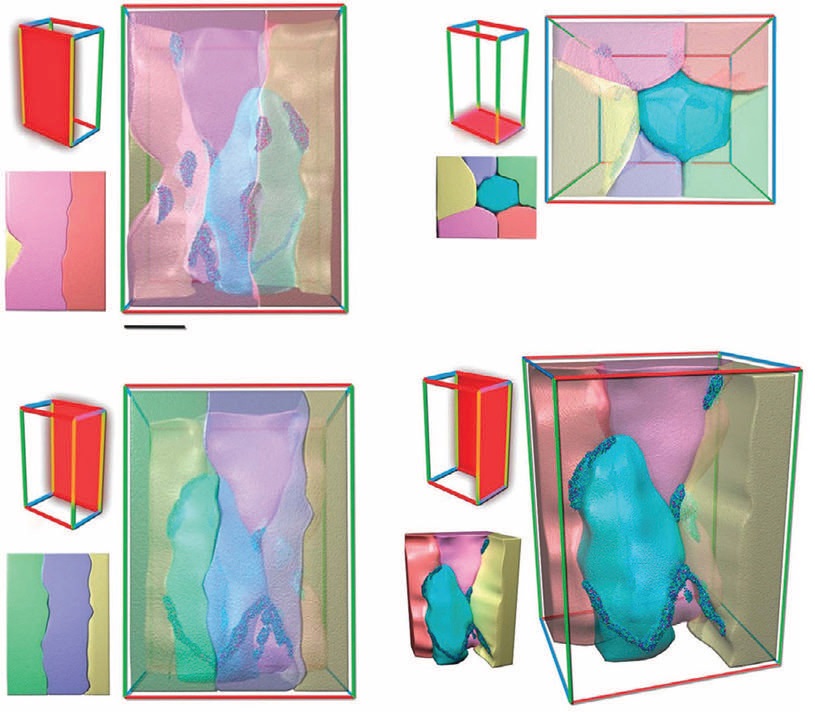 Figure 1. Atom probe tomography of various views of NH-7075 is shown. The different colors show aluminum and the other atoms (copper, silicon, chromium and titanium) present in the alloy. (Courtesy of North Carolina State University)
Figure 1. Atom probe tomography of various views of NH-7075 is shown. The different colors show aluminum and the other atoms (copper, silicon, chromium and titanium) present in the alloy. (Courtesy of North Carolina State University)
Zhu indicates that theoretically any age-hardening alloy can be strengthened through the use of current approach described in this article. He says, “We are now in the process of applying this technique on magnesium, a much lighter metal than aluminum.”
The preparation of stronger aluminum alloys that do not sacrifice ductility should lead to the greater use of this metal in automotive and aerospace applications. Further information can be found in a recent article (
2) or by contacting Zhu at
ytzhu@ncsu.edu.
REFERENCES
1.
Canter, N. (2010), “Ductile Metallic Glasses,” TLT,
66 (6), pp. 16–17.
2.
Liddicoat, P., Liao, X., Zhao, Y., Zhu, Y., Murashkin, M., Lavernia, E., Valiev, R. and Ringer, S. (2010), “Nanostructural Hierarchy Increases the Strength of Aluminum Alloys,”
Nature Communications,
1 (63), doi: 10.1038/ncomms1062.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be submitted to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be submitted to him at neilcanter@comcast.net.