Presence of nanobubbles on superhydrophobic surfaces
Dr. Neil Canter, Contributing Editor | TLT Tech Beat August 2010
Using small angle X-ray scattering, researchers have devised a way to detect nanobubbles on superhydrophobic surfaces.
KEY CONCEPTS
•
Textured, superhydrophobic surfaces, which encourage the formation and stabilization of bubbles, were prepared and immersed in water.
•
X-ray scattering analysis shows direct evidence for the presence of nanobubbles on these surfaces.
•
The results show that water only penetrates about 5-10 nanometers into 24-nanometer-diameter cavities, about 15-30 layers of water molecules.
Improved wettability of lubricants on surfaces continues to be an objective for lubricant suppliers. They are striving to improve the ability of lubricants to adsorb on surfaces, which leads to better friction reduction and wear loss.
One surface type that has been looked at actively is superhydrophobic. In a previous TLT article, a superhydrophobic surface is defined as one in which water exhibits a contact angle of approximately 150 degrees (
1).
One of the key features of a superhydrophobic surface is that of water repellency. Water remains a major problem in lubrication systems and any means that can be done to keep it away from surfaces helps extend the life of the system.
Dr. Antonio Checco, associate scientist at the Brookhaven National Laboratory in Upton, N.Y., says, “One of the perceived reasons for the extreme water repellency of superhydrophobic surfaces is the presence of small gas pockets near the surfaces. These bubbles further reduce the amount of surface area that can be in contact with water, thereby increasing the macroscopic contact angle.”
The presence of surface texture encourages the formation of these bubbles. Checco says, “Texturing of surfaces with topographical features such as cavities promotes the formation and stabilization of bubbles. From a thermodynamic standpoint, coalescing of small bubbles into larger ones is encouraged. This particular effect is more likely to occur on flat as opposed to textured surfaces because in the latter case the bubbles are tightly confined within the surface textures.”
On superhydrophobic surfaces that exhibit textures on the nanometer scale, nanobubbles are expected to form. Firm evidence of the presence of nanobubbles would be helpful to better determine how these nanotextured surfaces repel water. However, nanobubbles on superhydrophobic surfaces have not been directly probed until now.
X-RAY SCATTERING
Checco and his coworkers devised an approach to confirm the existence of nanobubbles of gas on superhydrophobic surfaces. The researchers developed a textured surface and then immersed it in water. Nanobubbles were detected through the use of small angle X-ray scattering (SAXS).
The textured surface was prepared through a block copolymer, self-assembly-based fabrication technique. Checco says, “We started with a surface of flat, silicon wafers similar to the material used in the electronics industry. Subsequently, a thin film of a block copolymer consisting of polystyrene and poly(-methyl methacrylate) was deposited on the silicon surface.”
The block copolymer was annealed at 180 C in a vacuum oven causing a microphase separation to occur. This heating leads to the formation of poly(-methyl methacrylate) domains within a polystyrene matrix. Checco adds, “The result is the creation of poly(-methyl methacrylate) cylinders that are perpendicular to the silicon substrate.”
Treatment with ultraviolet light leads to the degradation of the poly(-methyl methacrylate) cylinder blocks and the crosslinking of the polystyrene. Checco says, “Ultraviolet light breaks bonds between the polystyrene and the poly(-methyl methacrylate). With the degradation of the poly(-methyl methacrylate), cylindrical cavities are formed that are 24 nanometers in diameter, 40 nanometers apart and arranged in a hexagonal lattice.”
These cavities are “transferred” onto the silicon surface through an etching process. The right image in Figure 3 is a scanning electron micrograph of the nanocavities.
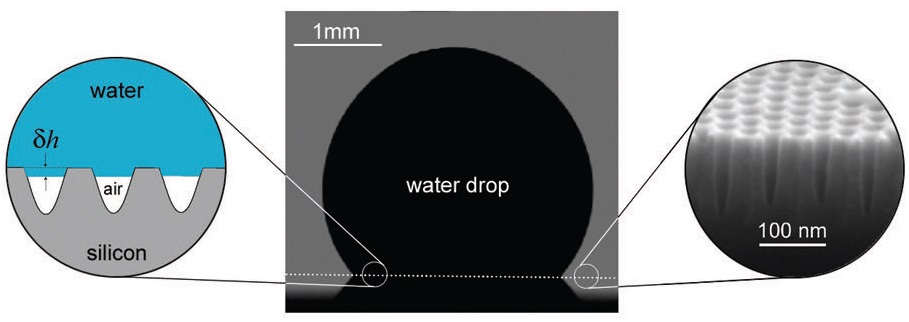 Figure 3. The image on the left is a cartoon showing the shape of a nanobubble inferred from x-ray measurements. The center image is an optical profile of a water drop placed on a nanotextured surface and the image on the right is a scanning electron micrograph of the nanocavities (Courtesy of Brookhaven National Laboratory)
Figure 3. The image on the left is a cartoon showing the shape of a nanobubble inferred from x-ray measurements. The center image is an optical profile of a water drop placed on a nanotextured surface and the image on the right is a scanning electron micrograph of the nanocavities (Courtesy of Brookhaven National Laboratory)
To render the surface superhydrophobic, the silicon surface was passivated with a 2.5-nanometer-thick monolayer of octadecyltricholorsilane. Ultrapure water was then placed between the textured surface and a thin Mylar film. The depth of the water layer was approximately 100 microns.
SAXS was used to detect the presence of the nanobubbles. Checco says, “We used SAXS because the size of the cavities in the textured surface is small enough to scatter x-rays. SAXS was used to compare the difference between scattered x-ray intensity in air and when water is placed on top of the surface.”
In analyzing the results, the researchers noted a reduction in scattered x-ray intensity when water is used. Checco says, “Water is denser than air and should generate less scattered x-ray intensity if it penetrates into the cavities. The results show that the scattered x-ray intensity is higher than expected if water penetrates completely into the cavities. This strongly indicates that water penetration is only partial and nanobubbles are present in the remainder of the cavities.”
Specifically, the researchers found that water only penetrates about 5 to 10 nanometers into the cavities, which corresponds to approximately 15 to 30 layers of water molecules.
The left image in Figure 3 is a cartoon illustrating the nanobubbles’ shape as inferred from x-ray measurements. In the center image, the optical profile of a water drop placed on the nanotextured surface is shown.
The scattered x-ray intensity measurements were taken at various time intervals to evaluate the stability of the nanobubbles. Checco says, “We found that the values remained constant, which means that the nanobubbles are stable.”
Confirmation of the existence of nanobubbles on superhydrophobic surfaces means that they clearly contribute to water repellency. One potential application for utilizing nanobubbles is in reducing the friction encountered by micro- and nanofluids as they flow into small channels.
Further information can be obtained in a recent article (
2) and by contacting Checco at
checco@bnl.gov.
REFERENCES
1.
Canter, N. (2008), “Controlling Surface Wettability,” TLT,
64 (5), pp. 12–13.
2.
Checco, A., Hofmann, T., DiMasi, E., Black, C. and Ocko, B. (2010), “Morphology of Air Nanobubbles Trapped at Hydrophobic Nanopatterned Surfaces,”
Nano Letters,
10 (4), pp. 1354–1358.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.