Heat-resistant nanoparticles
Dr. Neil Canter, Contributing Editor | TLT Tech Beat July 2010
Researchers develop a new technique to stabilize nanoparticles at elevated temperatures.
KEY CONCEPTS
•
Nanoparticles do not exhibit adequate thermal stability needed to function as heterogeneous catalysts in applications such as automotive emissions.
•
Platinum-rhodium bimetallic nanoparticles have been developed that operate at higher temperatures.
•
The mechanism for how these nanoparticles function is bleeding of lower melting platinum from the nanoparticles in a sacrificial self-stabilization process.
Nanoparticles are finding useful applications in lubricants as these small particles can impart such characteristics as improved antiwear and extreme pressure characteristics. But there remain a number of issues that need to be overcome for industry to more effectively work with nanoparticles.
In a previous article, a new technique was discussed to better determine the actual size of nanoparticles (
1). The method, known as induced grating, enables the size of nanoparticles to be measured down to 0.5 nanometers. Particles are measured accurately by taking advantage of the fact that smaller particles diffuse faster than larger ones.
An area of active research interest for nanoparticles is as heterogeneous catalysts in such applications as the production of fuels and reduction of automotive emissions. The small size of these catalysts leads to increasing surface-to-volume ratios that can enhance activity and effectiveness.
But these processes require catalysts to be stable at temperatures above 600 C. Goetz Veser, associate professor & CNG faculty fellow in the chemical engineering department at the University of Pittsburgh in Pittsburgh, says, “While promising as catalysts, nanoparticles do not demonstrate adequate thermal stability at high temperatures. Most nanoparticles are stable up to temperatures between 150 C and 250 C. At temperatures in the range between 500 C and 600 C, nanoparticles start to sinter, which leads to agglomeration and the formation of larger particles. This becomes a major problem at a temperature of about 800 C.”
Veser explains that nanoparticles exhibit this property due to simple thermodynamics. He says, “The total energy of a system is equal to the bulk plus the surface energy. Small nanoparticles are more energetic than larger ones. In thermodynamics, there is a natural drive toward a lower energy state, which is accomplished through agglomeration of the smaller particles into larger ones.”
This effect can be seen very dramatically in nanoparticles, which are five nanometers in diameter and smaller. For metals, the melting point of nanoparticles less than five nanometers drops dramatically. The depression in melting point is traced directly to the small nanoparticles and the larger contribution of surface energy.
Capping agents that are organic in nature were tried in attempt to stabilize the nanoparticles. Veser says, “The capping agents are simple ionic and nonionic surfactants that act as a surface barrier around the nanoparticles.” Unfortunately, this technique only has a slight impact as these surfactants are typically not stable at elevated temperatures either and nanoparticles, hence, can remain stable only up to temperatures around 400 C.
A different approach is needed to stabilize nanoparticles at elevated temperatures. Such a technique has not become available until now.
SACRIFICIAL SELF-STABILIZATION
Veser and his research associate, Anmin Cao, found that enhanced thermal stability can be achieved through the preparation of platinum-rhodium bimetallic nanoparticles on a barium hexa-aluminate support. Veser says, “We developed platinum-rhodium nanoparticles with a narrow size distribution centered at four nanometers that did not agglomerate in heating up to a temperature of 850 C. Instead, we found that the less stable platinum content bled away from the bimetallic nanoparticles. This process in effect generated bimetallic particles enriched with the higher melting point metal, rhodium.”
In heating up the bimetallic nanoparticles, platinum started to bleed away from the nanoparticles at a temperature of approximately 700 C. Veser considers this process to be comparable to the lower melting platinum distilling away from the bimetallic nanoparticles in a sacrificial self-stabilization process.
Veser indicated that typically platinum nanoparticles will start to degrade at temperatures between 550 C and 650 C. If higher levels of rhodium are added, then the stability can rise to between 700 C and 800 C.
This sacrificial self-stabilization was evaluated by assessing the performance of the catalyst in a methane combustion reaction. Veser says, “We found that the bimetallic catalyst performed well with ignition temperatures as low as 450 C and complete conversion at 580 C. The average size of the nanoparticles remained at approximately four nanometers, even during extended exposure at 850 C. In fact, high activity was still observed at 950 C, even though most of the platinum content had agglomerated to larger particles at that temperature.”
Veser points out that the large platinum particles that bled away did not contribute significantly or hinder the activity of the bimetallic nanoparticles.
Transmission electron microscopy was used to examine the nanoparticles and verify their stability at elevated temperatures. Figure 2 shows a high resolution image of two bimetallic nanoparticles that were heated to 700 C. The crystalline structure seen indicates that the larger particle consists of platinum, which has bled from bimetallic nanoparticles while the smaller one is a stable bimetallic platinum-rhodium nanoparticle.
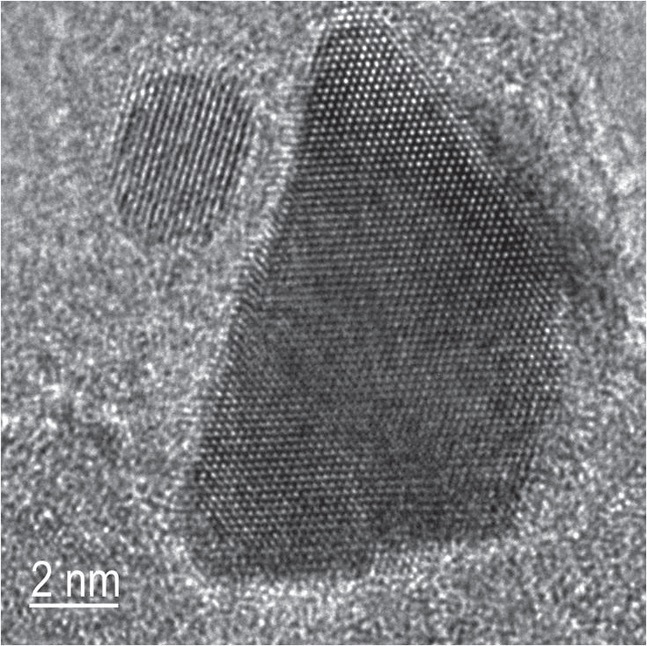 Figure 2. Greater thermal stability of nanoparticles was achieved by the bleeding of platinum from a smaller platinum-rhodium bimetallic nanoparticle on the left to a larger particle consisting exclusively of platinum on the right. (Courtesy of the University of Pittsburgh and reprinted by permission of Macmillan Publishers Ltd., Nature Materials)
Figure 2. Greater thermal stability of nanoparticles was achieved by the bleeding of platinum from a smaller platinum-rhodium bimetallic nanoparticle on the left to a larger particle consisting exclusively of platinum on the right. (Courtesy of the University of Pittsburgh and reprinted by permission of Macmillan Publishers Ltd., Nature Materials)
Veser indicates that the ratio of platinum to rhodium used in preparation of the bimetallic nanoparticles does not matter. He says, “It appears that the sacrificial self-stabilization enables the nonmetallic particles to self-adjust in order to retain stability regardless of the starting ratio of the raw materials.”
The importance of this work is that nanoparticles can now be developed that will remain stable and catalytically active under conditions needed to prepare fuels and reduce emissions. Veser believes that this approach should be applicable to other multimetallic nanoparticle catalysts.
Future work is focused in two areas. Veser is looking to add the bled platinum back to the bimetallic nanoparticles. He says, “We are trying in effect to glue the tail back on.”
A second aspect is to try to trick thermodynamics by setting up a nano-confinement process to completely frustrate the bleeding mechanism. In this way, Veser hopes to learn more about the mechanism of the process.
Further information can be found in a recent article (
2) or by contacting Veser at
gveser@pitt.edu.
REFERENCES
1.
Canter, N. (2010), “Measuring Nanoparticles,” TLT,
66 (1), pp. 14–15.
2.
Cao, A. and Veser, G. (2010), “Exceptional High-Temperature Stability through Distillation-Like Self-Stabilization in Bimetallic Particles,”
Nature Materials,
9 (1), pp. 75–81.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.