Gas-expanded lubricants
Dr. Neil Canter, Contributing Editor | TLT Tech Beat May 2010
A liquid carbon dioxide enables end-users to adapt these materials to different real-world operating conditions.
KEY CONCEPTS
•
Gas-expanded liquids are mixtures of a gas such as carbon dioxide and a solvent under elevated pressures.
•
One major benefit for potentially using gas-expanded liquids in lubricants is the ability of the end-user to adjust the properties of the mixture to meet changes in operating conditions.
•
Initial work with 15% carbon dioxide in polyalkylene glycols in a modeling study has shown a reduction of 20% in power loss in a journal bearing.
Gas-expanded liquids have been widely studied in the chemical sector for a variety of applications since the 1990s. According to Andres Clarens, assistant professor of civil and environmental engineering at the University of Virginia in Charlottesville, Va., “These liquids are often a mixture of a gas such as carbon dioxide and a solvent under elevated pressures. By controlling the pressure and the temperature of the mixture, it is possible to carefully specify the properties of the fluid, which has important implications in a lot of applications where tunability is desired.”
These mixtures make for easy separation of carbon dioxide from a solvent. Clarens indicates that one other benefit is that these mixtures reduce the total amount of solvent required for a specific operation. He says, “Adding carbon dioxide reduces the amount of solvent required for a specific application, and this lowers the cost and reduces the environmental footprint.”
But Clarens indicates that these mixtures have only taken off in the pharmaceuticals sector and other high-value applications such as nanoparticle formations and in the recycling of homogeneous catalysts. Part of the reason is that high-pressure equipment is needed that can withstand pressure of a few megapascals.
Lubrication is an area that may be receptive to this approach. In a previous TLT article, a new metalworking fluids technique known as Advanced Minimum Quantity Lubrication (AMQL) was described (
1). Solid carbon dioxide particles are combined with a metalworking fluid and then this mixture is treated with a propellant such as carbon dioxide gas. The mixture is delivered at speeds greater than 200 meters per second to combine lubricity with very efficient cooling.
Clarens has initiated a project to extrapolate the gas-expanded liquid concept to lubricants. He says, “One of the reasons for my interest is that I conducted research in graduate school on the use of supercritical carbon dioxide in metalworking fluids.”
Initial work shows that gas-expanded lubricants demonstrate great promise as shown below.
JOURNAL BEARING MODELING STUDY
Clarens envisions that introduction of liquid carbon dioxide into a lubricant will provide the end-user with the ability to adjust the mixture’s properties to meet changes in operating conditions. He adds, “Most conventional lubricants are developed to meet specific applications, but their properties cannot be altered if changes occur in the operation unless the lubricant is removed. Use of liquid carbon dioxide will enable the end-user to change physical properties such as viscosity to adapt the lubricant to different real-world operating conditions.”
An initial system evaluated by Clarens involves the use of carbon dioxide in polyalkylene glycols (PAGs). He says, “We picked this lubricant because of past work done using PAG-based lubricants in refrigeration applications. Slight changes in the carbon dioxide content can lead to huge changes in the viscosity of the gas-expanded lubricant.”
A gas-expanded lubricant has the appearance of a homogeneous liquid with the carbon dioxide dissolved in the mixture. Carbon dioxide exhibits a viscosity slightly lower than water. This means that addition of even 1% to 2% carbon dioxide can rapidly reduce the viscosity of the mixture. One of the main advantages of using liquid carbon dioxide under slight pressure is that, if the viscosity of the lubricant must be increased, the pressure can be reduced and the carbon dioxide will separate out as a gas, restoring the properties of the original base lubricant.
Clarens found this effect with PAGs of varying viscosities. The value of using carbon dioxide is especially apparent if the viscosity of a specific lubricant needs to be reduced by adding a higher level of the liquid.
A better understanding of the impact that carbon dioxide has on the performance of a PAG lubricant was obtained through a modeling study on a journal bearing operation. This modeling approach was conducted on an ISO 32 PAG fluid that is treated with 5% and 15% carbon dioxide. The results are compared to a petroleum oil-based ISO 32 fluid and to the untreated PAG lubricant.
The key parameter measured is power loss. Addition of 15% carbon dioxide into the PAG lubricant led to the largest reduction in power loss for bearings operated between 3,000 and 14,000 rpm. Overall, Clarens reports that the modeling study indicates that a 20% reduction in power loss is achievable using gas-expanded lubricants.
The differences between using a conventional lubricant and a gas-expanded lubricant are shown in Figure 1 for the bearing system. Additional elements needed for using a gas-expanded lubricant are a gas reservoir and a purge valve, which is used to adjust gas concentration in the lubricant. Gas can be added or purged depending upon the demands placed on the lubricant by the bearing system.
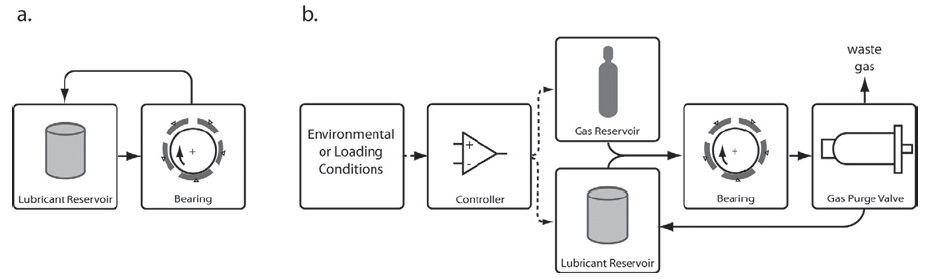 Figure 1. The contrast between using a conventional lubricant in a bearing system (a.) and a gas-expanded lubrication system (b.) is shown. A gas reservoir and a purge valve are used to adjust the gas concentration in the lubricant. (Courtesy of the University of Virginia)
Figure 1. The contrast between using a conventional lubricant in a bearing system (a.) and a gas-expanded lubrication system (b.) is shown. A gas reservoir and a purge valve are used to adjust the gas concentration in the lubricant. (Courtesy of the University of Virginia)
Clarens finds that other synthetic basestocks such as polyalphaolefins and polyol esters can be used to prepare gas-expanded lubricants with carbon dioxide. But petroleum oil has proven to be more of a challenge. He says, “Petroleum oil is a mixture containing high and low molecular weight molecules. Carbon dioxide is not compatible with all of these components, and some separation is seen.
Clarens believes that a lubricant needs to be as aliphatic and as homogeneous as possible to be compatible with carbon dioxide. Additives used in lubricant formulations will also have to be evaluated.
With the entrainment of a gas in the lubricant, foam might be anticipated to be a concern. Clarens comments, “We do not believe foam will be a problem, as we have sheared gas-expanded lubricants in studies we have done at high rates. But accelerated oxidation of the lubricant could be an issue.”
Future work will involve the preparation of a rotor-test bed in which fluid film bearings are floated in the gas-expanded lubricant to go beyond the results of the modeling study and do some actual testing.
Information on the initial work done by Clarens can be found in an upcoming presentation that has been submitted for publication (2). Clarens can also be contacted at
aclarens@virginia.edu.
REFERENCES
1.
Canter, N. (2007), “Machining with AMQCL technology,” TLT,
63 (7), pp. 14–15.
2.
Wang, S. and Clarens, A. (2010), “Feasibility of Gas-Expanded Lubricants for Increasing Energy Efficiency in Power Turbines,”
Preprint Paper-American Chemical Society, Division Fuel Chemistry,
55 (1), Submitted for publication.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.