Rapid dispersion of particles
Dr. Neil Canter, Contributing Editor | TLT Tech Beat April 2010
These solids are capable of dispersing quickly when placed in a liquid medium.
KEY CONCEPTS
•
Solid particles quickly disperse when placed into a liquid medium.
•
This phenomenon occurs because the particles pick up energy after displacing water from the air-water interface. This acquired energy enables the particles to rapidly oscillate, leading to the generation of repulsive hydrodynamic forces that cause the fast dispersion.
•
Smaller particles disperse faster than larger ones. As a result, the speed of dispersion increases as the number of particles dropped into a liquid also increases.
Solid lubricants such as molybdenum disulfide and polytetrafluoroethylene are very effective at reducing friction and increasing the load-carrying capacity of lubricants. Due to the insolubility of these materials in oil and water, introducing them into liquid lubricants has proved to be a challenge.
This problem is overcome by placing the solid lubricants into stable dispersions in either oil or water. Typically, the concentration of the solid lubricant in the dispersion can range from 10% up to 35%.
With the increasing interest in nanotribology, researchers are looking to also find carriers or solvents to utilize these small nanoparticles in liquid lubricants. A recent TLT article discussed the development of a dispersion of nanoparticles of potassium borate in an ester matrix (
1). This borate-based lubricant additive can be used in oil- and water-based lubricants.
But not much is known about why solid particles quickly disperse when introduced into a liquid medium. Figure 2 shows the process as a solid material such as flour introduced into water. Images are taken 0.033 seconds, 0.161 seconds and 0.363 seconds after the solid is added to the water shows the rapid rate of dispersion.
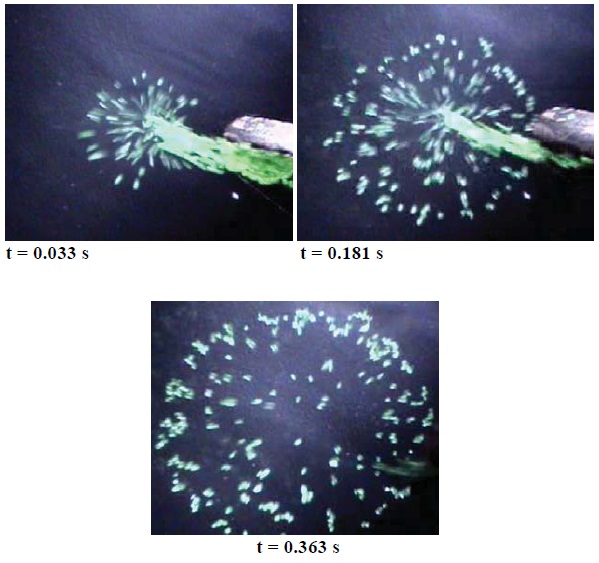 Figure 2. The dispersion process is so explosive that after 0.363 seconds the solid has moved through the liquid. (Courtesy of the New Jersey Institute of Technology)
Figure 2. The dispersion process is so explosive that after 0.363 seconds the solid has moved through the liquid. (Courtesy of the New Jersey Institute of Technology)
Pushpendra Singh, professor of mechanical engineering at the New Jersey Institute of Technology in Newark, N.J., says, “This is an experiment you can do at home in your kitchen by filling a dish with water, waiting a few minutes for the water to become still and then adding a solid such as flour or finely crushed pepper.”
Singh notes that a few milligrams of a solid can fill the entire surface of the water almost instantaneously. The reason for why particles react in this violent fashion has not been known until now.
REPULSIVE HYDRODYNAMIC FORCE
Singh and his researchers determined that the rapid dispersion of particles is due to the development of repulsive hydrodynamic forces that arise because, as the particles are adsorbed at the interface, they move rapidly in the direction normal to the liquid surface. Glass and mustard seed particles ranging in diameter from 10 microns to 1.1 millimeters were dropped into water, corn oil and glycerin. The velocity of the particles was measured through the use of a video camera.
Singh says, “Once a solid particle comes into contact with a liquid, it is pulled down into the liquid by a force due to the interfacial tension and establishes an equilibrium position of the particle at the interface between the liquid and the air. The extent to which the particle goes below the surface is dependent upon its weight, the interfacial force and the contact angle of the air-liquid interface.”
For example, Singh notes that a particle with a contact angle of 90 degrees must move downwards by a distance equal to its radius, assuming it is spherical. The particles will then oscillate up and down about their equilibrium height at the interface. He adds, “The particle oscillates in a similar fashion to an under-damped spring-dashpot system. The frequency of this oscillation for a mustard seed with a diameter of 1.2 millimeters is approximately 83 Hertz.”
This process leads to a decrease in the interfacial energy. Singh explains, “The decrease in the energy is due to the decrease in the area of the air-water interface, a part of which is replaced by the particle. A large fraction of the released interfacial energy is transferred to the particle.”
As the particle rapidly oscillates to dissipate the acquired energy, it forces the liquid around it to move away and give rise to repulsive hydrodynamic forces that cause particles to disperse. Singh says, “Particle velocity at the air-water interface can be as high as 47 meters per second (or 168 kilometers per hour).”
The speed of the particle, assuming all other parameters are fixed, is dictated by its size, according to Singh. As the radius decreases, the mass decreases to a much larger extent (decreases as the third power of the radius) than the interfacial energy acquired by the particle (decreases as the square of the radius), which means that smaller particles disperse at a faster rate than larger ones.
Fluid viscosity plays a factor in slowing down the speed of the particle. Singh says, “As the fluid viscosity increases, it acts to reduce the particle’s speed, which then lowers the rate of dispersion.” The researchers found that speed of the dispersion of glass particles in water is three times faster than in a solution of 60% glycerin in water. The latter exhibits a viscosity that is six times higher than water.
An interesting aspect of particle dispersion is that the speed of the particles increases as the number of particles dropped into a liquid also increases. Singh explains, “Repulsive hydrodynamic forces that arise as two particles are adsorbed near each other at the interface are stronger, because each particle creates an outward flow and the net sum of the flow is higher than for an individual particle. This result means that the rate of dispersion will increase, even more as the number of particles increases above two.”
Singh indicated that this phenomenon was determined not only through experimentation but also by the use of direct numerical simulation. When a cluster of particles is dispersed, the researchers found that the particles moved in a radial direction away from the center, as seen in Figure 2. The final radius reached by the particles also increases as a function of the number of particles.
The chemical structure of the particle and its polarity with respect to the liquid medium do not impact the rate of dispersion, but the addition of a surfactant can reduce dispersion speed significantly. Singh says, “Surfactants adsorbed in the interface act to reduce surface tension. This effect leads to a reduction in the energy that can be transferred to individual particles, thus reducing the rate of dispersion.”
Singh found that even using soap to wash the lab equipment used in the experiments adversely impacted the rate of dispersion. He says, “We had to clean the glassware without using surfactants in order to not affect the results.”
Further information can be found in a recent paper (
2) or by contacting Singh at
singhp@njit.edu.
REFERENCES
1.
Canter, N. (2009), “Boron Nanotechnology-Based Lubricant Additive,” TLT,
65 (8), pp. 12–13.
2.
Singh, P., Joseph, D., Gurupatham, S., Dalal, B. and Nudurupati, S. (2009), “Spontaneous Dispersion of Particles on Liquid Surfaces,”
Proceedings of the National Academy of Sciences,
106 (47), pp. 19761–19764.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.