Unleashing the potential of ionic liquids
Jean Van Rensselar, Contributing Editor | TLT Cover Story April 2010
These under-researched materials have unusual properties that could make them an ideal lubricant choice.
KEY CONCEPTS
•
Additive-enhanced ionic liquids show great promise as lubricants.
•
Extremely versatile ionic liquids are simple and relatively inexpensive to develop.
•
While research is complex, the future is bright.
Trying to study ionic liquids is pretty much like trying to:
•
Herd highly caffeinated cats.
•
Babysit two-year-old triplets.
•
Spin plates on sticks.
•
Eat bread while talking.
•
Drive while texting on the Autobahn.
In other words, it’s not easy. However, it’s really more like trying to take care of two-year-old triplets than herding cats—if you’re successful, the rewards are substantial.
Those involved in IL research are wowed by the possibilities and—at the same time—frustrated by the complexities. While all new disciplines suffer from lack of coordination and focus, this is especially true with ILs because of the enormous range of options and the fact that they’re relatively easy to formulate.
While IL lubricant research is very new, ILs are currently in use in chemical synthesis and separation, food science, cellulose processing, petroleum processing, paint formulation, nanomaterial processing, nuclear energy, solar energy, hydrogen storage, waste recycling and battery manufacture.
IL tribology studies have looked at interfaces including aluminum-steel, steel-steel, steel-copper, steel-SiO
2, Si
3N
4-SiO
2 and silicon wafers—with mostly encouraging results. These studies and others conclude that the many benefits of using IL lubricants include:
•
Reduced parasitic energy loss by reducing friction.
•
Extended service life and maintenance cycles because of wear reduction.
•
Expanded high temperature lubricant usage because of high thermal stability.
•
Safer transportation and storage because of non-flammability.
In addition, ILs don’t evaporate like most other liquids, one of the reasons they hold so much promise as lubricants.
STLE-member Dr. Jun Qu, R&D staff scientist at Oak Ridge National Laboratory in Oak Ridge, Tenn., who is working with IL lubricant formulation, says, “Basically these lubricants can improve and replace many lubricants that are currently being used with potential friction and wear reductions up to 50%. But people need to understand that ionic liquid lubricants are fundamentally very different than existing lubricants, both from a chemistry perspective and a performance point of view.”
THE DEFINITION
An IL is pretty much a liquid salt made up of ions and ion pairs. More specifically, “ionic” refers to inorganic salts with high melting points. The term ionic liquid, introduced to avoid using the ambiguous term “molten salts” and possibly for patent purposes, refers to thermally stable molten salts composed of asymmetrical cations and anions that melt below 100 C and are capable of liquefying at room temperature. They are also called RT-ILs (room-temperature ionic liquids).
Both the cation and anion control the chemical, physical and lubricating properties. Some of the most common cations are imidazolium, phosphonium, pyridinium and ammonium. Common anions include BF
4, PF
6, CF
3SO
3 and N(CF
3SO
2)
2.
CURRENT RESEARCH
Research is vigorous but topically and geographically diffuse. Of the published scientific papers, about 35% have been published by European authors, 30% by U.S. authors, 20% by Chinese authors and 10% by Japanese authors.
A number of studies, using primarily X-ray photoelectron spectroscopy (XPS) to examine surface interactions, are underway to unlock the lubrication potential of ILs.
Dr. Maria-Dolores Bermúdez, professor of materials science and engineering at the Polytechnic University of Cartagena, Spain, is at the center of IL research. She reports that they’ve been studying ammonium and imidazolium ILs as titanium lubricants. While one type of imidazolium IL decomposes at the metal-to-metal contact, another forms adsorbed layers on the titanium surface and a phosphate-containing protective layer on the steel counterface.
The most studied light alloys for ILs are steel and aluminum—mostly because of their wide range of applications in sliding components—especially in the auto industry.
Because of surface interactions through the phosphate atoms, new imidazolium ILs with lateral phosphate groups are showing promising tribological performance as neat lubricants for those contacts.
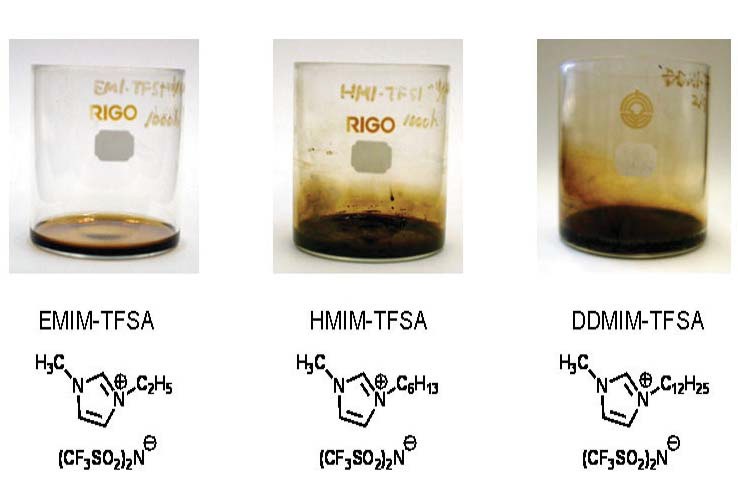 Figure 1. Thermal stressed samples (200 C, 1,000 hours) (Courtesy of Idemitsu Kosan Co., For further details, see Journal of Synthetic Lubrication, (2007), 24(3), pp. 135-147)
Figure 1. Thermal stressed samples (200 C, 1,000 hours) (Courtesy of Idemitsu Kosan Co., For further details, see Journal of Synthetic Lubrication, (2007), 24(3), pp. 135-147)
Although imidazolium ILs are the most studied family of IL lubricants, other cations also are under review, with encouraging results for ammonium derivatives.
STLE-member Dr. Ichiro Minami, associate professor of chemistry at Iwate University in Morioka, Iwate, Japan, says, “Fundamental academic research and applied industrial research are ongoing in Japan. However only a little information has been published, and most of the results weren’t disclosed.”
In order to address the secrecy issue, the Japanese Society of Tribologists organized a technical committee to share information about tribological applications for ILs. Minami is the chair.
“Ionic liquids are of interest mainly for electrochemists and for synthetic chemists in Japan,” Minami adds.
Dr. W. Robert Carper, professor emeritus of chemistry at Wichita State University, in Kansas, explains, “There is very little IL tribological research in the U.S. now. Most of the work is being done in China, Japan and Europe—including Spain, Germany, France and Ireland—and there is also some in South America.
“There has never been much in the way of research money spent on ionic liquids in the U.S. In Europe, they are getting both applied and basic research money. Most of the funding here has been for basic research, and very little of it has gone into ionic liquids, which is unfortunate because it looks like from an industrial point of view they are one of the waves of the future.” Carper is very active in computer modeling of IL tribology in reference to a variety of metallic and ceramic surfaces.
Carper’s recent work highlights the importance of IL surface interactions between IL anions and metallic surfaces—ceramic interactions involve both IL cations and anions.
THE BIRTH OF IONIC LIQUIDS
No one really knows when the first ionic liquid was discovered or who discovered it. But other milestones are clear.
•
1941: Latvian-German chemist Paul Walden synthesized one of the earliest known room-temperature ILs.
•
1961: The title of the Faraday Society discussion held in Liverpool was “The Structure and Properties of Ionic Melts.”
•
1992: J.S. Wilkes and M.J. Zaworotko formulated an air and water stable IL that was based on the tetrafluoroborate anion. Those stable ILs were the first selected as lubricants.
•
2001: Weimin Liu and colleagues created interest in IL lubricants by studying alkyl-imidazolium tetrafluoroborates in a wide range of contact materials.
•
2005: The first conference on ionic liquids took place in Salzburg.
If the number of published papers is any indication, the field of IL lubricants is rapidly expanding. A Sci-Finder survey conducted by Carper indicates that the first three were published in 2001 and, by mid-December, there were 29 for 2009. Another source puts the 2009 figure at 35.
 Figure 2. Dr. Ichiro Minami of Iwate University in Japan evaluates the tribological properties of a novel lubricant.
FORMULATIONS
Figure 2. Dr. Ichiro Minami of Iwate University in Japan evaluates the tribological properties of a novel lubricant.
FORMULATIONS
Among the oddities of these designer fluids are neat vs. additive properties. Neat ILs may provide more significant friction reduction than IL additives, while a low proportion of IL additives may contain enough IL molecules to form stable adsorbed layers on the surface to reduce wear as effectively.
Neat ILs. As neat lubricants, ILs establish a tribolayer that is physically adsorbed onto and/or chemically reacted with the metal surfaces to effectively reduce friction and wear under boundary lubrication.
But in some circumstances, when the two contact surfaces are reactive to each other, neat ILs may not be appropriate because of their electrical conductivity.
ILs as additives. Because of their organization in polar and nonpolar domain solutions and miscibility with polar and nonpolar solvents, ILs have been studied as lubricating additives in water and lubricating oils.
When added to water, ILs reduce the initial period of high friction in ceramic-ceramic sliding contacts. When added to grease, ILs substantially improve performance, indicating a synergy with the additives present in the formulated grease.
When used as mineral oil additives, the more effective wear-reducing Ils are those with shorter alkyl chains. When used as synthetic oil additives, all IL additives at 100 C reduce both friction and wear of the base oil.
Recently dicationic bis(imidazolium) ILS with the same long side-chain substituted cation and different anions were evaluated as additives in polythene glycol at room temperature. Results showed that they could effectively reduce the friction and wear of steel-steel sliding pairs better than the base oil without additives.
The excellent tribological properties of ILs as additives are due to the:
•
Formation of physically adsorbed films, similar to a friction modifier.
•
Formation of tribochemical products during friction, creating an antiwear boundary film.
Basic properties of Ionic liquids
Base: A salt with a large cation or anion
Freezing Point: Generally below room temperature (25 C)
Liquid State Limit: Often over 200 C
Thermal Stability: High
Viscosity: A very large range from single digit to over 1000 cP at room temperature
Solvent/Catalyst Capability: Excellent for organic reactions
Vapor Pressure: None to very low
Dielectric Constant: Less than or equal to 30
Polarity: High (cations and anions instead of neutral molecules)
Molar Conductivity: Less than 10 Scm2 mol-1
Specific Conductivity: Good, less than 10 mScm-1
Electrochemical Window: 2.0V - 4.5V
The addition of 1% TCP and 1% IL to base oil establishes a substantial tribofilm and reduces wear volumes better than 1% TCP alone or 1% IL alone. Although the evidence of synergy between ILs and TCP is clear, the nature of the relationship is an area for further study.
ADDITIVES FOR ILS
Although many cost-effective lubricant additives are available, most were developed for mineral oils and won’t dissolve in ionic liquids. Saturated aliphatic compounds generally don’t mix well with ILs, but olefins do better and aldehydes do quite well. When it comes to gases, hydrogen is slightly soluble, carbon monoxide is better and carbon dioxide does well.
When it comes to improving wear and friction properties of ILs with additives, the purity of the base IL is extremely important—with a highly purified IL reducing friction about five times better than a reagent-grade IL.
To reduce the corrosivity of more reactive ILs—especially those containing fluorine anions—research has focused on adding wear and corrosion reducers. Benzotriazole is attractive because its molecular structure is similar to that of IL. Studies show that it can appreciably reduce corrosion and wear.
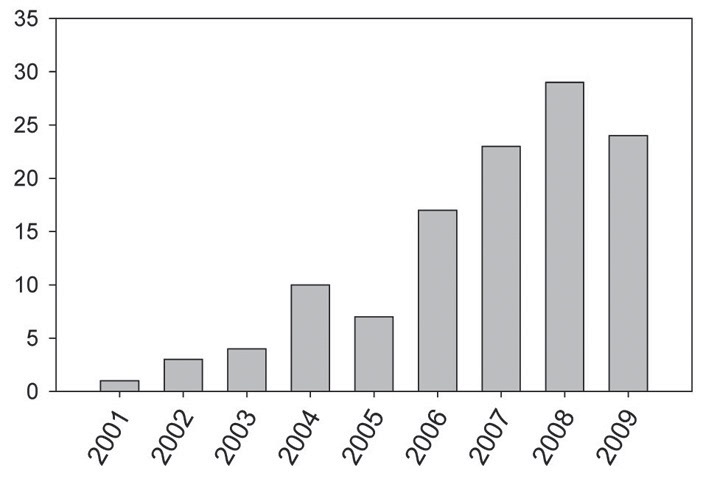 Figure 3. Evolution of the number of scientific papers published on the use of ionic liquids in lubrication. (Source: Bermúdez, M.-D., Jimenez, A.-E., Sanes, J. and Carrion, F.-J. (2009), “Ionic Liquids as Advanced Lubricant Fluids,” Molecules, 14(8), pp. 2888-2908)
Figure 3. Evolution of the number of scientific papers published on the use of ionic liquids in lubrication. (Source: Bermúdez, M.-D., Jimenez, A.-E., Sanes, J. and Carrion, F.-J. (2009), “Ionic Liquids as Advanced Lubricant Fluids,” Molecules, 14(8), pp. 2888-2908)
Minami and colleagues identified the first additives that improve the friction and wear properties of ionic liquids—specifically tetra-alkylammonium and tetra-alkylphosphonium salts of N-protected aspartic acid that were dissolved in 1-alkyl-3-methylimidzolium bis(trifluoromethylsulfonyl) imide.
When compared to the same test for neat IL or neat TCP, the addition of 1% TCP to ionic liquids rapidly established a tribofilm and reduced the wear volume by 64%.
“Prototype additives can enhance the antiwear properties of ionic liquids,” Minami says. “Similar to mineral and synthetic fluids, ionic liquids have to be optimized by additive technology in order to meet the requirements of practical applications.”
Addressing commercialization barriers
Following are recommendations taken from the BCS, Inc., report: “Accelerating Ionic Liquid Commercialization (
1).”
•
Develop new efficient process designs that minimize the volume costs of IL systems.
•
Demonstrate IL performance with pilot-plant or semi-commercial scale projects.
•
Determine the impact on human health with early stage toxicology tests and establish use guidelines.
•
Assess environmental impacts for ILs and their breakdown products.
•
Perform economic benefits analyses for using ILs in select industries.
•
Perform focused R&D to synthesize ILs that meet important industrial parameters– increased lifetime, reduced costs, reduced toxicity and increased contamination tolerability.
•
Develop robust and economic scale-up methods for industrial use of ILs.
•
Demonstrate reproducible production of high purity, high quality ILs.
•
Develop strategic partnerships among industry stakeholders to accelerate commercialization through exchange of information and data on current and developing processes.
1. Full report Accelerating Ionic Liquid Commercialization, available here.
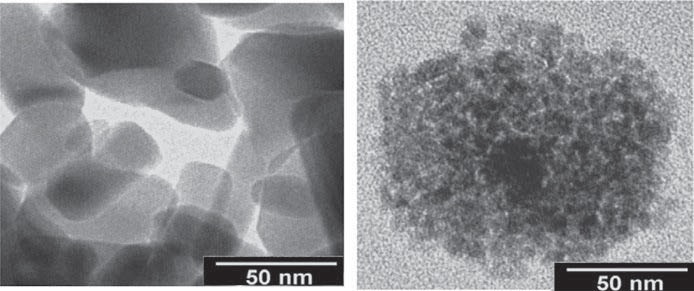 Figure 4. TEM micrographs of Neat ZnO nanoparticles (shown on the left) and IL-modified ZnO nanoparticles within PC matrix (shown on the right). (Source: Bermúdez, M.-D., Jimenez, A.-E., Sanes, J. and Carrion, F.-J. (2009), “Ionic Liquids as Advanced Lubricant Fluids,” Molecules, 14(8), pp. 2888-2908)
GOOD NEWS/BAD NEWS
Figure 4. TEM micrographs of Neat ZnO nanoparticles (shown on the left) and IL-modified ZnO nanoparticles within PC matrix (shown on the right). (Source: Bermúdez, M.-D., Jimenez, A.-E., Sanes, J. and Carrion, F.-J. (2009), “Ionic Liquids as Advanced Lubricant Fluids,” Molecules, 14(8), pp. 2888-2908)
GOOD NEWS/BAD NEWS
Many of the unique traits of ILs make them either desirable or undesirable, depending on the application. For example, the downside of flexibility is complexity; the downside of water miscibility can be decomposition. Following are some of the more important properties.
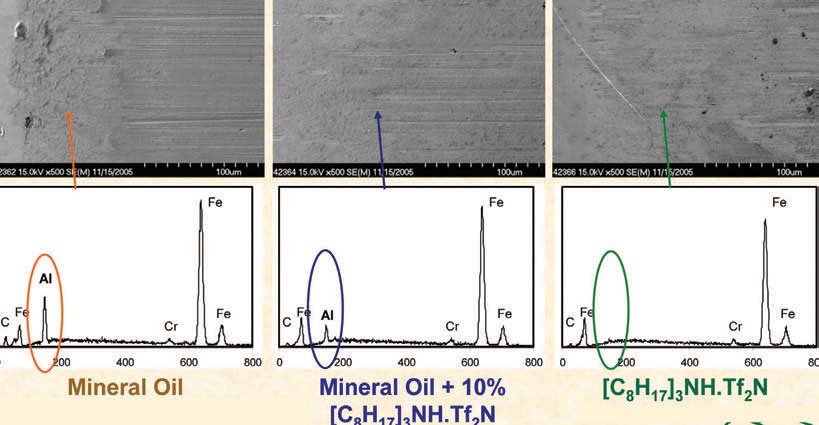 Figure 5. Images of steel counterface contact areas.
Flexibility.
Figure 5. Images of steel counterface contact areas.
Flexibility. The possible formulations are nearly endless—somewhere in the range of a million. Properties such as melting point, viscosity, acidity, density and hydrophobicity can be adjusted by simple changes to the anion-cation structure. This easy customization is why ILs are sometimes referred to as designer liquids.
“Designing and synthesizing ionic liquids is no longer a major problem,” Carper explains. “Frankly, if you can figure out what type of chemical group or unit you need to use, through measurements and some modeling, you can make an appropriate ionic liquid rather easily. In most cases, it’s a matter of synthesizing or purchasing a cation and an anion and simply putting them together. That’s not really very hard anymore. There are large numbers of articles on the synthesis of ionic liquids. In Europe they’re producing ionic liquids for chemical reactors by the tons.”
The downside of the flexibility issue is that the research is extremely diffuse and thus complicated.
Miscibility/water sensitivity. Depending on the structure, some ILs are highly miscible with water and others are hydrophobic. And because ILs are so easy to alter, this is beneficial for solvent extractions or product separations where the relative solubility of the ionic and extraction phase can be adjusted to facilitate separation and recycling.
On the other hand, when water is added to an ionic liquid or not completely removed:
•
Some cations irreversibly decompose.
•
Water may not bind strongly to one of the ions.
•
Water may dissolve the IL until it forms a saturated salt solution.
Hydrophobic anions create more stability than hydrophilic anions.
Low vapor pressure. Most ILs have little or no vapor pressure. In addition to preserving the lubricant and preventing the contamination of machine components, this prevents the escape of potentially toxic fumes. Low vapor pressure is an especially important property in aerospace applications with high vacuum conditions and requirements for high load-carrying capacity.
Thermal-oxidative stability. The heat generated by friction during machine operation causes thermo-oxidative degradation, which leads to undesirable changes in the physical and chemical properties of lubricants and creates volatile fragments. The thermo-oxidative stability of ILs, which depends on the structure of both the anion and the cation, is generally much greater than conventional synthetic lubricants.
Specifically, alkylimidazolium-derived ILs are much more stable than synthetics. The addition of additives further improves thermal-oxidative stability.
In general, imidazolium-based dicationic liquids have higher degradation temperatures (>400 C) than their triazolium analogues. Because dicationic ILs display excellent tribological traits at 300 C, they have potential as high-temperature lubricants.
Combustibility-flammability. Most ILs are nonflammable, making them a highly desirable choice for many applications. However, some ILs’ decomposition products formed on heating are combustible and require careful handling.
Environmental issues. Because ILs have almost no vapor pressure, they don’t emit volatile organic compounds. Researchers have been moving away from the more toxic materials such as halogenated compounds and toward new anions such as bistriflimide. There is also a growing movement toward less toxic cations such as ammonium salts.
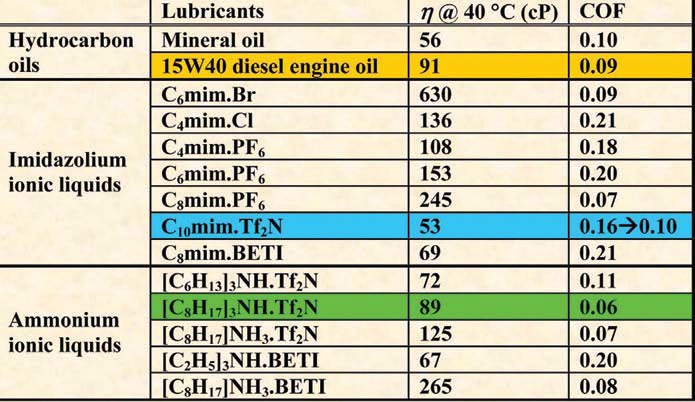 Figure 6. Friction screening tests.
Figure 6. Friction screening tests.
“Determining environmental toxicity requires work,” Carper says. “Some of the larger companies are already looking into that, and there are many articles on toxicity. With the low vapor pressure, there isn’t much to worry about, but in handling ionic liquids, you might have to deal with an unusually toxic compound. You’d have to do the necessary tests—to see if it’s carcinogenic, for example. But from an environmental contamination standpoint, there’s not much to dispose of. Depending on how benign they are, you can break down most ionic liquids with chemical reactions.”
Research into aquatic toxicity shows that some ILs are as toxic as most substances developed for the same uses. And in the case of ILs, mortality isn’t the most important aquatic metric. Sublethal concentrations can still have dire effects on aquatic organisms. This is another area for study.
ILs often can be recycled—which increases both environmental and price appeal. “One of the advantages of using ionic liquids is that they can, in most cases, be recycled over and over again,” Carper says.
Reducing the friction in IL lubricants
According to Dr. Maria-Dolores Bermúdez, professor at the Polytechnic University of Cartagena in Spain, there are a number of ways to reduce IL lubricant friction, but additives are key. She explains:
In order to understand the complex surface chemistry taking place at the materials’-IL interfaces, most of the reported tribological works contain detailed surface interaction studies by XPS (X-ray photoelectron spectroscopy), particularly in the case of ceramic and metal lubrication with imidazolium hexafluorophosphates and tetrafluoroborates.
The general process that takes place includes oxidation, dissociation of the anions, formation of metallic phosphates, oxides and fluorides and precipitation of ceramic phases such as boron oxide, boron carbide and boron nitride. Decomposition of the imidazolium cation with formation of nitrogen oxides and organometallic species also has been proposed. During the wear process, nascent metallic surfaces are produced which could enhance the metal-IL reactivity and favor the process of IL decomposition.
When the formation of bridge complexes between the surfaces and the IL molecules is favored with electron-donor atoms, the adsorbed IL layers are more stable and protect the surface.
For effective lubrication, these stable adsorbed tribolayers must combine with the presence of flexible units. The length of these units is a critical parameter in the antiwear properties since it determines the polarity of the IL.
PRICE
One of ILs’ strongest selling points is that they’re simple and relatively inexpensive to produce. IL reactions can be carried out with no special apparatus or methodology. In fact, the reactions often are quicker and easier to complete than those in conventional organic materials. But the poor economy of small batches makes them expensive to utilize.
“Tribologists recognize that ionic liquids are expensive,” Minami says. “While many researchers have found advantages with ionic liquids, their cost/performance is prohibitive.” Since the field of ILs is so new, it’s difficult to make long-term price predictions. But as far as synthesis goes, Carper expects the cost of ILs to be competitive with commercial synthetic oils soon. Right now, the uncertainty of IL lubricant cost and availability makes ILs less attractive to industrial users.
“It’s a little expensive when you start synthesizing a new compound, but once the compounds are ramped up, the cost goes down considerably,” Carper says. “I looked at the price recently of certain compounds and saw that people can buy a ton of certain types of ionic liquids for as little as 50 British pounds (about $82 U.S. dollars). If you get something that’s a winner, you should be able to upscale it without going broke.”
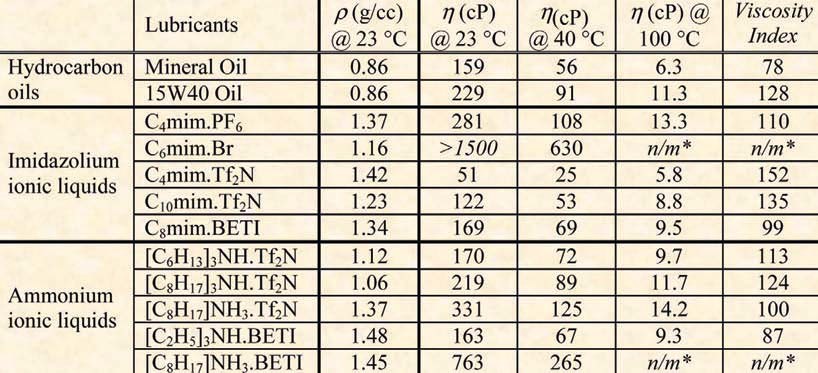 Figure 7. Ionic liquid viscosity ranges.
PROMISING APPLICATIONS
Figure 7. Ionic liquid viscosity ranges.
PROMISING APPLICATIONS
“IL lubricants are currently not in commercial use yet, basically because they are so new,” Qu explained. “Another reason they aren’t being used is that right now the current market for ionic liquids is so small that it makes the price very high—even though the actual synthesis cost is relatively inexpensive.”
There has been growing IL research interest in recent years because of unusual properties including high thermal-oxidative stability, non-volatility, non-flammability, high ionic conductivity and miscibility with organic compounds.
“These unique properties make ILs strong candidates as lubricants, especially for high-performance lubricants where thermal stability and non-volatility are the prerequisites,” Carper says. “ILs have been verified as versatile lubrication oils for different sliding pairs and exhibit high friction reduction, antiwear performance and high load-carrying capacity. They also can be used in the form of ultrathin films.”
Regarding the auto industry, a group of ammonium ILs have been developed with encouraging lubrication performance. When compared with 15W40 diesel engine oil in laboratory bench tests, these ILs exhibit:
•
A surface-boundary film believed to reduce friction and wear.
•
Up to 35% less friction and up to 55% less wear in lubricating steel-aluminum contacts.
•
Up to 55% less friction and up to 21% less wear in lubricating Cr-plated piston rings against cast iron.
A major goal of current tribological research is to find effective lubricants for reactive light alloys such as magnesium, aluminum and titanium, which are of particular interest in the auto industry because of their lower densities and the ability to form corrosion-protective surface layers.
Because of their excellent thermal and electrical conductivity, ILs are perfect for micro/nano-electromechanical (MEMS/NEMS) systems. Research on MEMS devices coated with a thick film of IL showed significant improvement in wear life.
Since ILs have inherent properties that are particularly advantageous for aerospace applications—such as low vapor pressure and high thermal stability—aerospace researchers are very interested.
Also, because they are nonflammable, ILs make sense in applications where fire risk is significant such as in combustion engines and machining processes and chemical, petroleum and aircraft industries.
 Figure 8. Piston ring-on-flat reciprocating sliding test.
THE BOTTOM LINE
Figure 8. Piston ring-on-flat reciprocating sliding test.
THE BOTTOM LINE
The study of IL lubricants continues to expand, mainly because they are so versatile and there are so many combinations yet to be developed.
Researchers believe that IL lubricants will be discovered that ideally suit high-vacuum, extreme-temperature and high-pressure applications. Ultimately, ILs won’t be able to compete in the marketplace until researchers solve the stability, toxicity and cost issues. Areas that call for further study include:
•
The environmental, safety and health of IL development, handling and implementation.
•
Economic benefits (a thorough analysis).
•
The feasibility of a central IL research facility.
•
The formation of protective tribofilms as they relate to surface interactions and tribochemical reactions between ILs and sliding counterparts.
•
The synergistic effect and long-term stability of tribocorrosion-reducing IL additives.
•
The feasibility of IL lubricants in contact with cryogenic or high temperature materials.
•
Polymer lubrication with ILs and polymer-IL interactions in new nanocomposites.
Currently, the general consensus is that ILs make better additives than either bases or neat lubricants. But that may change quickly. Researchers in Europe, Asia and the U.S. are working hard to capitalize on the enormous potential of ILs and hope these designer fluids will eventually solve some of the toughest lubricating problems global industries face.
“Commercialization takes time,” Qu says. “Synthetic lubricants have been around for about 50 years, but they still have a small share of the lubricant market today. ILs may have a faster route due to their promising potential. In 5-10 years I’d like to see ionic liquid lubricants starting to get into the market and in 10-15 years grow into a bigger market share.”
FOR MORE INFORMATION
For comprehensive information on ionic liquids, Dr. W. Robert Carper recommends the compilation,
Ionic Liquids in Synthesis, published in 2007 by VCH and edited by Peter Wasserscheid and Tom Welton. This book does not cover the newly emerging field of ionic lubricants, though.
To read/download Dr. Ichiro Minami’s 2009
Molecules article, “Ionic Liquids in Tribology” visit
here.
To read two recent reviews covering the field of ionic liquid lubricants, visit
here and
here.
 Jean Van Rensselar heads her own communication/public relations firm, Smart PR Communications, in Naperville, Ill. You can reach her at Jean@SmartPRcommunications.com
Jean Van Rensselar heads her own communication/public relations firm, Smart PR Communications, in Naperville, Ill. You can reach her at Jean@SmartPRcommunications.com.