Facile olefination of aromatic derivatives
Dr. Neil Canter, Contributing Editor | TLT Tech Beat March 2010
An alternative technique can prepare carbon-carbon bonds and synthesize more substituted aromatic molecules.
KEY CONCEPTS
•
Derivatives of aromatic compounds are widely used in lubricants as antioxidants, corrosion inhibitors, detergents and emulsifiers.
•
A new synthetic technique has been developed to directly add olefins to aromatic molecules under relatively low temperatures and short time frames.
•
This process may provide a more efficient and economical way to develop new aromatic derivatives for use as lubricant additives.
A large number of diverse additive and basestock technologies are used in the preparation of automotive and industrial lubricants. Many of these components are organic molecules that have been prepared by standardized reaction processes for many years.
Derivatives of aromatic compounds such as benzene, xylene and cumene are used as lubricant additives. Among the examples are alkyl phenols that can be used as antioxidants or precursors for detergents used in automotive lubricants. Alkylbenzene sulfonates are another member of this class and are used as detergents, corrosion inhibitors and emulsifiers in both automotive and industrial lubricants. A third example is aromatic amines such as alkylated diphenylamines that are widely used as antioxidants in automotive lubricants.
Derivatization of basic aromatic molecules has been difficult to achieve because it is not very easy to replace a hydrogen on an aromatic ring with a carbon chain. Jin-Quan Yu, associate professor from The Scripps Research Institute in La Jolla, Calif., says, “The main method used currently is known as the Mizoroki-Heck reaction. This process involves the addition of an olefin to an alkyl or aromatic halide in the presence of a base and a palladium catalyst.”
The Mizoroki-Heck reaction has proven to be an indispensable tool in organic synthesis. It relies on the need to utilize an organic halide molecule as an intermediate. Yu says, “Extra steps are required to halogenate the substrate and then remove it. This can lead to excessive waste, both in terms of the labor and energy needed to conduct these steps.”
Yu also points out that positioning the halide group on the organic substrate in the right position represents a challenge. Depending upon the target molecule, additional reaction steps may be required just to synthesize it properly.
Direct olefination of aromatic derivatives is an alternative approach that has been examined since the 1960s. This technique has not been fully utilized because of problems with controlling selectivity, utilizing versatile substrates and the need to develop simple and practical reaction conditions.
There is need for a technique that improves upon direct olefination of aromatic derivatives yet complements the Mizoroki-Heck reaction. Such a technique has not been devised until now.
DIRECT METHOD
Yu and his research group have now shown that an olefin can be directly added to a diverse group of aromatic derivatives under simple reaction conditions. He says, “This direct approach to prepare carbon-carbon bonds in place of carbonhydrogen bonds is a key discovery that will enhance the ability to synthesize more substituted aromatic molecules.”
The researchers focused on using phenylacetic acid derivatives as the substrates because they are readily available and reasonably priced. Figure 3 shows the scheme for the reaction of a substituted phenylacetic acid with an olefin. A sodium salt (such as table salt—sodium chloride), a palladium (II) catalyst, amino acid ligand and oxygen are used in the process.
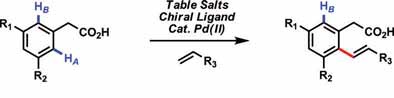 Figure 3. A direct olefination process has been developed that enables olefins to be easily inserted into aromatic ring systems under relatively mild conditions over a short time frame. (Courtesy of The Scripps Research Institute)
Figure 3. A direct olefination process has been developed that enables olefins to be easily inserted into aromatic ring systems under relatively mild conditions over a short time frame. (Courtesy of The Scripps Research Institute)
Yu says, “Two key discoveries have been achieved in our work. We found that a sodium salt can coordinate to the carboxylic acid group to accelerate the reactivity, and there is good selectivity to direct the olefin to a specific site on the aromatic ring. A second finding is that coordination of amino acid ligands to the palladium catalyst does not kill the reaction but, rather, controls the regiospecificity of the process.”
Without using the sodium salt, the reaction does not occur. From a selectivity standpoint, as the example in Figure 3 shows, the reaction conditions enable the olefin to become attached mainly in a position adjacent (or ortho) to the carboxylic group.
One of the more interesting aspects of this process is that the researchers found it was beneficial to run in the reaction in an oxygen environment. Oxygen works well as an oxidizing agent.
Benzoquinone also is employed to prevent a second olefin from being inserted onto the aromatic ring. Yu adds, “We want the process to be very selective and use benzoquinone to prevent the formation of difunctional species. This prevents the production of mixtures with the desired mono-functional adduct.”
Initial work to establish reaction conditions was conducted on 4-methoxyphenyl acetic acid and ethyl acrylate. Excellent yields were found when the reactants were heated at 90 C for 24 hours. Yu says, “These results met our goal of developing a process that is cost-effective and does not need to be run at temperatures above 100 C. In more recent work, we have evaluated a new amino acid ligand that is even more effective. We can achieve 100% yields when the reaction is run at a lower temperature (80 C) for only 20 minutes. Another benefit is only 0. 2% palladium is required.”
Yu indicates that this combination of reactants and conditions enables the process to work quite well. The researchers are still working to better understand the reaction mechanism.
Yu says, “We believe that the amino acid coordinates to the palladium complex, which then attaches to the olefin substrate and the phenylacetic acid species. We are still working on better understanding the transition state at this time.”
The researchers have used these reaction conditions to develop complex molecules that are intermediates in the manufacture of pharmaceuticals. Yu indicates that the researchers have now determined how to activate sp2 unsaturated species very well. The future challenge is to find ligands that can be used to react with saturated sp3 moieties.
This process provides a more efficient and economical way to insert unsaturated organic groups onto aromatic ring systems. It has the potential to provide lubricant additive suppliers with additional options to develop new molecules that may provide better performance in the future.
Further information can be found in a recently published article (
1) or by contacting Yu at
yu200@scripps.edu.
REFERENCE
1.
Wang, D., Engle, K., Shi, B. and Yu, J. (2010), “Ligand-Enabled Reactivity and Selectivity in a Synthetically Versatile Aryl C-H Olefination,”
Science,
327 (5963), pp. 315–319.
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.