Easy-to-clean oily surfaces
Dr. Neil Canter, Contributing Editor | TLT Tech Beat December 2009
A surface coating enables oil to be rinsed off a substrate without the use of cleaner.
KEY CONCEPTS
•
Removal of oil from metal surfaces is very difficult. Cleaners can be used but lead to problems with contamination and waste treatment.
•
A surface coating has been developed based on a perfluorinated technology that enables water to remove oil from metal surfaces.
•
The coating has been used to remove oils such as diesel fuel and olive oil.
In working with lubricants and greases, those of us in manufacturing plants face the challenge of removing excess oil from various surfaces. The oil may originate from leaking machines or misapplication.
Cleaning oil and grease from metal surfaces has always been problematic. Scrubbing just with water does not facilitate removal of the oil.
The use of cleaners is more helpful because they contain surfactants (also known as soaps) that can emulsify the oil. But the process can still be very labor-intensive and time-consuming. In addition, the used cleaners can present problems from contamination and waste treatment standpoints.
For example, leakage into a metalworking fluid system can cause the pH to rise above the normal range and also generate excessive foam. Both can cause great difficulty in managing such a system. Discharge of large quantities of cleaners into the effluent stream can lead to higher waste treatment costs needed to reduce such parameters as Biochemical Oxygen Demand (BOD) and Chemical Oxygen Demand (COD) down to the levels required by the local Publically Operated Treatment Works.
Jeffrey Youngblood, associate professor of materials engineering at Purdue University in West Lafayette, Ind., notes the difficulty with oil removal when skiing. He says, “Our interest stems from the time I was skiing and used my finger to clean off my goggles. Unfortunately, the fingerprint would not readily come off because oil from my skin adhered to the goggle’s surface, leading to the generation of fog.”
The fog develops because water present in the environment then beads up on the oily film. This phenomenon is very similar to the problem that occurs when trying to remove oil from a metal surface.
Efforts have been made to modify surfaces so that oil can be easily washed off. The polarity can be modified to range from being water compatible (hydrophilic) to oil compatible (hydrophobic). In a previous TLT article, researchers discussed the development of a surface that can transition from a superhydrophobic region to a superhydrophilic state (
1).
Use of either extreme surface does not lead to the easy removal of oil. Youngblood does not see the need to generate such severe conditions in order to remove oil from a surface. He says, “The best approach is to make sure that a surface is amenable to water wettability and sufficiently oleophobic so that oil can easily be removed.”
POLYMER BRUSHES
A surface coating has been prepared that can enable oil to be rinsed off a substrate without a cleaner. For glass, the initial coatings were prepared in a two-step sequence starting with the addition of an isocyanate-functionalized silane monolayer on the surface of this substrate. The isocyanate reacts with the terminal alcohol of a perfluorinated end-capped polyethylene glycol to form the coating.
Youngblood says, “Sufficient fluorine is present in the coating to produce a hydrophilic surface that will only allow water to penetrate and not oil-based materials. Polymer brush density is critical in enabling the coating to function properly.”
The brush nature of the coating stems from its appearance on the substrate. Youngblood explains, “As the polymer becomes denser and extends out from the substrate, it really starts to look like a brush.”
In a second generation of the technology, the researchers prepared copolymers based on fluorinated amphiphiles copolymerized with functionalized monomers that are designed to adsorb to specific surfaces. These copolymers easily can be added to surfaces through the use of solvent deposition.
Youngblood says, “We envision tailoring the molecular weight and the functional density for specific applications. A range of polymers is conceivable that can be used on metal, wood, plastic and cement surfaces. Our objective is to find the sweet spots of these substrates.”
Too low a density means insufficient fluorinated functionality is present to resist the deposition of oil. In contrast, too high a density will not allow water to penetrate to the surface.
The coating is found to adhere well to steel, copper and aluminum substrates. Some plastics such as polyethylene terephthalate and nylon, which are polar, also work. But problems have been encountered with polyolefins due to their non-polar nature.
Concern has arisen with using perflurorinated-based products in consumer applications. Youngblood indicates that his research group is looking at alternative chemistries such as perfluoropolyethers and silicones. He adds, “These options are not as good as perfluorinated end-groups but still may be acceptable.”
Several types of oils have been used in the coating evaluations. Youngblood says, “We used hexadecane as a direct comparison to diesel fuel, olive oil, motor oil and fingerprint grease. The coating was effective in enabling diesel fuel and olive oil to be removed by rinsing. Motor oil removal was not as easy, and semisolids such as greases are hard to remove.” Olive oil in particular was good to use because it is tinted green and removal can be easily seen.
Water quality also could be an issue. Youngblood indicates that distilled water was used primarily while tap water was tried only occasionally. Additional work needs to be done in this area.
Future work includes evaluating the potential for using the copolymers to coat filters that could be alternatives to reverse osmosis systems. Figure 2 shows how two drops of hexadecane can bead up on a glass filter modified with a copolymer containing fluorinated amphiphiles.
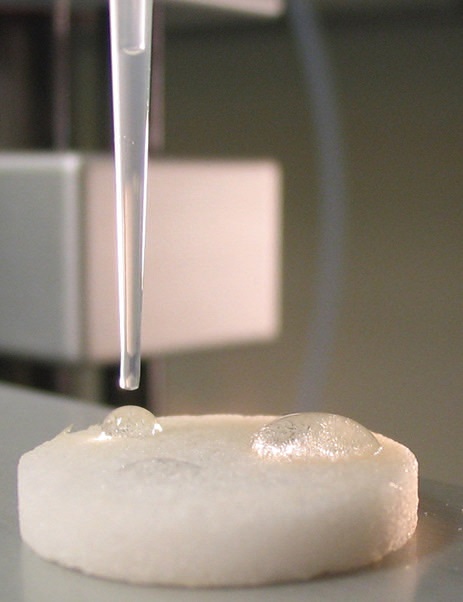 Figure 2. Copolymers based on fluorinated amphiphiles are finding use as coatings to enable oil to be easily rinsed from surfaces with water. Droplets of hexadecane bead up on a glass filter modified with a copolymer coating facilitating their removal. (Courtesy of Purdue University)
Figure 2. Copolymers based on fluorinated amphiphiles are finding use as coatings to enable oil to be easily rinsed from surfaces with water. Droplets of hexadecane bead up on a glass filter modified with a copolymer coating facilitating their removal. (Courtesy of Purdue University)
Additional information on the technology can be found in several recent references (
2-4). Youngblood can be contacted at
jpyoungb@purdue.edu.
REFERENCES
1.
Canter, N. (2007), “Wettability Gradient on a Surface,” TLT,
63 (8), pp. 16–18.
2.
Howarter, J. and Youngblood, J. (2009), “Amphiphile Grafted Membranes for the Separation of Oil-in-Water Dispersions,”
Journal of Colloid and Interface Science,
329 (1), pp. 127–132.
3.
Howarter, J. and Youngblood, J. (2008), “Self-Cleaning and Next Generation Anti-Fog Surfaces and Coatings,”
Macromol. Rapid Commun.,
29 (6), pp. 455–466.
4.
Howarter, J. and Youngblood, J. (2007), “Self-Cleaning and Anti-Fog Surfaces via Stimuli-Responsive Polymer Brushes,”
Adv. Mater.,
19 (22), pp. 3838–3843.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.