Aluminum hydride: Potential hydrogen-storage material
Dr. Neil Canter, Contributing Editor | TLT Tech Beat November 2009
In addition to automobiles, the new process could be used in superconductors and batteries.
KEY CONCEPTS
•
Identification of a suitable hydrogen-storage material continues to be a major challenge because DOE is requiring a system with a mass of 6% hydrogen and a volume of 45 kilograms of hydrogen per cubic meter by 2010.
•
Aluminum hydride (alane) has the hydrogen content and volume to meet DOE’s requirements but is difficult to manufacture.
•
A new electrochemical process conceivably can generate hydrogen from alane for powering an internal combustion engine. The alane can be regenerated through a recycling procedure that improves the efficiency of the process.
Research is continuing developing more viable ways to store hydrogen for use in fuel cell propulsion systems. Hydrogen by nature is very explosive and difficult to handle. Use of a derivative with a high hydrogen content that has excellent stability is required, particularly because the consumer will need to purchase it in a similar fashion to gasoline.
A past column discussed the preparation and isolation of a series of polyhedral aluminum hydrides (
1). One of the derivatives, Al4H6, has been found to exhibit the best stability and could be a suitable hydrogen-storage material.
The U.S. Department of Energy has a goal of developing a hydrogen-storage system with a mass of 6% hydrogen and a volume of 45 kg of hydrogen per cubic meter by 2010. The objective increases to a system with a mass of 9% hydrogen and a volume of 81 kg per cubic meter by 2015. Aluminum hydride or alane is a potential candidate because it contains 10% hydrogen by weight and has a volume of 149 kg of hydrogen per cubic meter.
Ragaiy Zidan, advisory scientist at the DOE’s Savannah River National Laboratory, says, “Alane is a viable candidate to store hydrogen. Although it is not stable thermodynamically, it is stable kinetically. The hydrogen release from alane is easier than from most other high-capacity hydrogen-storage materials.”
But Zidan maintains that the conventional process used to prepare this hydride is impractical. Alane has traditionally been prepared by reaction of lithium alanate with aluminum chloride in diethyl ether solution. The process generates an ether complex of aluminum hydride. Heating under vacuum conditions leads to the isolation of alane.
Zidan comments, “This process has several problems including that it is highly exothermic and people shy away from using diethyl ether as a solvent. The biggest problem is that the byproduct, lithium chloride, acts as an energy sink. An enormous amount of energy needs to be used to melt lithium chloride and electrolyze the melt in order to reverse the process and yield the corresponding metal needed to complete the cycle.”
Melting of lithium chloride needs to occur at a temperature above 600 C and requires 429 kilojoules per mole of energy, equivalent to the heat of formation and fusion of the salt to recover the lithium. Direct reaction of aluminum with hydrogen is also impractical because it must be done under high level of hydrogen pressure (105 bars) at room temperature.
Development of a more efficient and safer process would potentially enable alane to store hydrogen. Such a process has not been developed until now.
ELECTROLYSIS
A new approach has been developed to utilize alane as a source of hydrogen and then regenerate it in a cyclical manner. Zidan says, “A breakthrough achieved in 1998 found that the process for preparing sodium aluminum hydride is reversible. We took this information and developed an electrochemical process in which sodium aluminum hydride dissolved in the solvent tetrahydrofuran (THF) is used as the electrolyte.”
The alane adduct with THF is generated at the anode through the reaction of alanate ion (AlH4-) with aluminum metal. This can result in the consumption of an aluminum electrode, as shown in Figure 3.
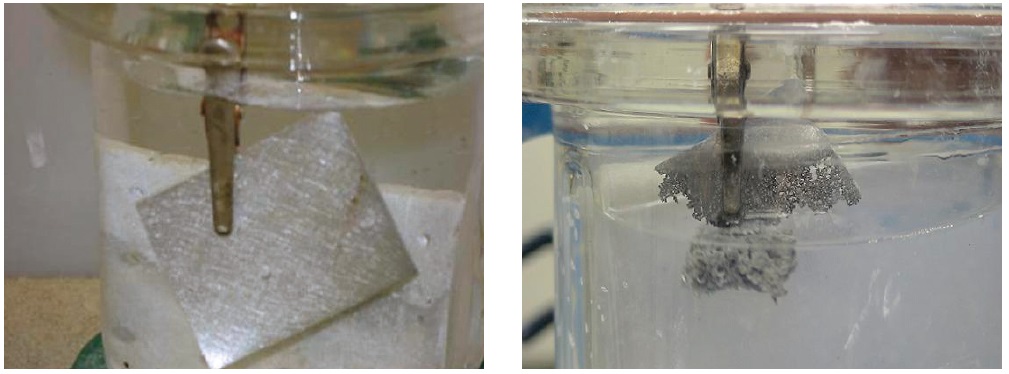 Figure 3. The electrochemical process for using alane as a source of hydrogen can consume an aluminum electrode. The image on the left shows the aluminum electrode prior to the reaction of the alanate ion in THF. The image on the right shows the aluminum electrode consumed after the reaction. (Courtesy of the Savannah River National Laboratory)
Figure 3. The electrochemical process for using alane as a source of hydrogen can consume an aluminum electrode. The image on the left shows the aluminum electrode prior to the reaction of the alanate ion in THF. The image on the right shows the aluminum electrode consumed after the reaction. (Courtesy of the Savannah River National Laboratory)
Alane then can release hydrogen using the heat generated in an internal combustion engine to drive the reaction. Concurrently, a sodium cation is reduced at the cathode and can be readily treated with hydrogen gas to form sodium hydride.
Aluminum metal formed in this process is recycled by reaction with sodium hydride in the presence of a titanium catalyst. The result is the regeneration of sodium aluminum hydride for continuing use of alane as a source of hydrogen.
There is sufficient hydrogen formed to both power the automobile and react with the sodium cation. Zidan says, “Stored hydrogen is typically released during the breakdown of alane at a temperature between 100 C and 150 C. The temperature can be lowered by controlling the particle size of the alane powder. The temperature required for the release process should not drop below 100 C because hydrogen can leak out of the alane.”
One step that proved to be difficult was the isolation of alane from the THF adduct. Zidan says, “We added triethylamine to the process, which facilitated the formation of an adduct with alane. Free alane was then isolated by heating the adduct under vacuum conditions.”
This research demonstrates the feasibility of using alane in a reversible process to generate a stable hydrogen derivative suitable as a storage material that can readily release the fuel at the right time. In addition, recycling can be done to regenerate the hydrogen-storage material using a process that is efficient and does not involve dealing with thermodynamically unfavorable byproducts.
Zidan believes this process can be used in other applications such as batteries and semiconductors. Additional information can be found in a recent article (
2) or by contacting Zidan at
ragaiy.zidan@srnl.doe.gov.
REFERENCES
1.
Canter, N. (2006), “Introducing a New Class of Hydrides,” TLT,
63 (6), pp. 20–21.
2.
Zidan, R., Garcia-Diaz, B., Fewox, C. and Stowe, A. (2009), “Aluminum hydride: A Reversible Material for Hydrogen Storage,”
Chem. Comm.,
25, pp. 3717–3719.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.