Nanoparticle-based emission catalysts
Dr. Neil Canter, Contributing Editor | TLT Tech Beat November 2009
A new process more effectively and efficiently prepares automotive catalysts to maximize performance.
KEY CONCEPTS
•
Effectiveness of the three-way automotive catalyst is reduced because precious metals tend to migrate along the surface and agglomerate into larger particles.
•
A direct-current plasma process has been developed to prepare fine precious metal nanoparticles that range from 2-4 nanometers. This catalyst exhibits superior performance over a longer operating period.
•
The nanosized precious metal catalyst can work at a lower treat rate, representing a 50% cost savings in precious metals.
Reducing automotive emissions increases the need for more effective catalyst technologies. In the U.S., EPA has reduced the NO
x emission requirements to 1. 2 grams per brake hp hour in 2007. This emissions requirement will drop further to 0.2 gram per brake hp hour in 2010.
In a past column, the benefits of a cerium oxide-modified Cu-ZSM-5 catalyst were described in reducing NO
x emissions (
1). The introduction of cerium oxide enabled the catalyst to function in the temperature range (between 325 C and 350 C) of diesel exhaust. The catalyst can remove 95% to 100% of NO
x emissions.
The conventional way to reduce emissions in gasoline engines is to use a socalled three-way catalyst that relies primarily on precious metals (palladium, platinum and rhodium). In this approach, the three main pollutants (carbon monoxide, hydrocarbon and NO
x) are reduced simultaneously. Maximum benefit for the catalyst is obtained by adjustment of the ratio of air to fuel.
For diesel engines, a DOC, or diesel oxidation catalyst, is typically employed consisting primarily of platinum and palladium. Max Biberger, CEO of SDCMaterials Inc., in Tempe, Ariz., says, “Tightening requirements for fuel economy and the use of turbochargers has led to a reduction in the temperature of diesel emissions. This factor increases the challenge in removing pollutants because catalyst performance declines as the temperature drops. Catalyst effectiveness at lower temperatures can only be improved by adding more precious metals which, unfortunately, leads to added cost.”
A wet chemical process is the standard currently used for DOC and three-way catalyst fabrication to place precious metals on an oxide surface.
Biberger says, “Use of a wet chemical process is not effective in securing precious metal catalysts on the surface. Due to the wetting angle, the precious metals tend to migrate along the surface, which can lead to agglomeration into larger particles. The result is a loss of catalyst effectiveness.”
A process needs to be developed to more effectively and efficiently prepare automotive catalysts in order to maximize performance. Such a process has not been developed until now.
DC PLASMA
Better utilization of the precious metals is achieved by a patented process for converting the starting materials into nanosized catalysts. Biberger explains, “We have developed a direct-current (DC) plasma process that transforms the precious metal starting materials (typically with particle sizes in the micrometer range) into fine nanoparticles that form the basis for the catalyst.”
Biberger indicates that the key to catalyst performance is that the nanosized particles fabricated using this DC plasma methodology do not change their size over a long operating period. He adds, “There is no loss of surface area as the precious metal nanoparticles do not move around and agglomerate.”
The nanoparticles derived from the DC plasma range between 2-4 nanometers, which is no different from the conventional wet chemical process. But catalyst prepared by the later procedure are more mobile on the oxide surface leading to agglomeration and loss of surface area.
Biberger says, “We employ a similar ratio of precious metals as compared to conventional processing but can use less catalyst because aging is reduced. The catalyst chemistry also can be adjusted to meet specific applications.”
Testing of the catalysts prepared by the DC plasma process was conducted through direct comparison with catalysts used in commercial automobiles. Biberger says, “We have focused on evaluation against catalysts used in Volkswagen automobiles because this car company is the largest manufacturer of light-duty diesel engines and, in our view, has the most advanced technology.”
Biberger indicates that his company buys original equipment catalytic converters for Volkswagen cars such as the Golf and the Passat, removes and analyzes the catalyst components and develops a catalyst with comparable properties. Testing is conducted by an independent laboratory. A typical procedure is to test the catalyst for the equivalent of 100,000 miles at a temperature of 800 C and a water concentration of 10% for 16 hours.
The light off temperature (T
50) for removal of carbon monoxide, hydrocarbons and NO
x is measured during testing. This parameter is an indication of the temperature at which a catalyst achieves a 50% conversion rate. Biberger adds, “The goal is to build a catalyst with less precious metal that can convert at lower temperatures over the expected service life.”
Figure 1 shows two catalysts developed by the DC plasma technique in comparison to a Volkswagen reference catalyst. The key results for all three emissions categories are shown in yellow. The Volkswagen catalyst exhibits initially lower T
50 values, but the T
50 figures are much higher when aged.
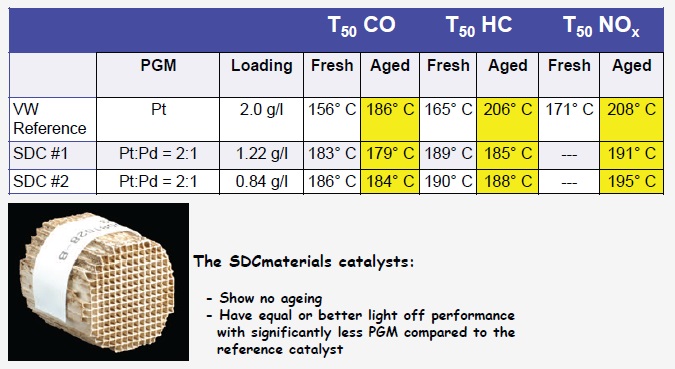 Figure 1. The light off temperature (T50) for removal of carbon monoxide, hydrocarbons and NOx is measured for two catalysts developed by the DC plasma technique and compared to a reference Volkswagen catalyst. The lower temperature values for the DC plasma catalysts show that they will operate more cost-effectively over a longer service life. (Courtesy of SDCMaterials, Inc.)
Figure 1. The light off temperature (T50) for removal of carbon monoxide, hydrocarbons and NOx is measured for two catalysts developed by the DC plasma technique and compared to a reference Volkswagen catalyst. The lower temperature values for the DC plasma catalysts show that they will operate more cost-effectively over a longer service life. (Courtesy of SDCMaterials, Inc.)
Biberger figures there is typically 5 grams of precious metal per liter of catalyst in most cars. The DC plasma catalyst can work at a lower treat rate, which will represent a 50% cost savings in precious metals. This could mean an annual savings of as much as $4 to $7 billion.
Looking ahead, Biberger indicates that his company is developing so-called Non-PGM catalysts, or Non-Platinum Group Metal catalysts, i.e., catalysts that contain no precious metals. He figures a commercial, non-PGM product is still a couple of generations away.
On the gasoline side, there is not as much of an incentive to move to new catalyst technologies because precious metal material cost is lower by 50% compared to diesel engines.
Biberger says, “There is only a little room for improving the cost of catalysts used in gasoline engines. However, we are making progress in developing an approach that could be available soon.”
Further information on the preparation of diesel engine catalysts using DC plasma technology is found in a recent U.S. Patent Application (
2). Biberger can be reached for additional information at
max.biberger@sdcmaterials.com.
REFERENCES
1.
Canter, N. (2007), “Catalyst Eliminates Diesel NOx Emissions,” TLT,
63 (8), pp. 12–14.
2.
Biberger, M. (2008), “Nano-Skeletal Catalyst,” U.S. Patent Application
2008/0280756 A1.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.