Special Report: Boundary Lubricity Additives
Dr. Neil Canter, Contributing Editor | TLT Tech Beat September 2009
An in-depth primer on defining this additive type, analyzing its properties and understanding how to make the best selection for your application.
 www.canstockphoto.com
www.canstockphoto.com
KEY CONCEPTS
•
Boundary lubricity additives are derived from fatty oils and their derivatives. These additives were the original lubricants used in the mid-1800s in the U.S. but have since taken a secondary role to petroleum oil basestocks.
•
Selection of a boundary lubricity additive is based on the operating conditions in a specific application.
•
Super-lubricity additives are a special type of boundary lubricity additives that can expand the range of operating conditions under which film lubrication is sustained and can work synergistically with antiwear additives.
•
A significant application for super-lubricity additives is to reduce the amount of ZDDP used in specific applications. Super-lubricity additives form a thick film that protects ZDDP.
In a past additive column we explored the different types of extreme pressure (EP) agents and provided information on future trends to enable readers to learn more about how to use this important class of additives (
1). We decided on the topic after surveying TLT readers.
Considering the variety of additives used to boost the performance of industrial lubricants, we decided to do another readership survey in mid-2009 to see what additive type our readers would most like to learn more about. The majority of readers said lubricity additives have the greatest impact on lubricant performance and is the area they are most interested in learning more about.
From the survey we determined there was some confusion about the differences between boundary lubricity additives and extreme pressure agents. In this article, TLT requested interviews with key manufacturers of boundary lubricity additives to obtain their insights on how these additives function, how to differentiate the performance of boundary lubricity additives, when to recommend a boundary lubricity additive instead of an EP agent and future trends.
In alphabetical order, the companies that agreed to be interviewed are Arizona Chemical, Croda Lubricants, E-ION Additives, Elevance Renewable Sciences, Inolex Chemical, Kyowa Hakko and Lubrizol. Initially, all respondents were asked to respond about how boundary lubricity additives are defined and how they function.
Our questions and their answers follow.
WHAT IS A BOUNDARY LUBRICITY ADDITIVE, AND HOW DOES IT FUNCTION?
The term “lubricity” brings to mind a fatty oil or grease that is derived from a natural source, be animal or vegetable (
2). The original lubricant used in the U.S. in the mid-1800s was oil obtained from sperm whales. In the 1850s, production of sperm-whale oil averaged more than 100,000 barrels/day (
3).
But the dominance of naturally derived lubricants did not last because petroleum oil became much more readily available in 1870 and has since become the main basestock used up to now. Fatty oils and their derivatives, whether naturally or synthetically derived, have now moved into the role of boundary lubricity additives that supplement petroleum oil by preventing two metal surfaces in close proximity from contacting each other. Such a role prevents increases in wear and friction.
STLE-member Dr. Boris Zhmud, R&D manager for EION Additives, says, “A boundary lubricity additive is any chemical component but the base oil itself that improves lubricating efficiency under boundary lubricant conditions that occur when the friction is largely determined by the surfaces and surface-chemical properties of the lubricant.” In making this comment, Zhmud differentiates boundary lubrication from hydrodynamic lubrication, which occurs when two surfaces are kept separate because of the viscosity of the lubricant.
STLE-member Joe Purnhagen, global commercial manager-metal processing additives for The Lubrizol Corp., says, “As the name implies, boundary lubricity additives reduce friction and wear by maintaining a physical boundary between contacting surfaces (generally in a liquid phase). In metalworking fluids, the term ‘lubricity additive’ is widely used, but the functionality is not unlike what is commonly referred to as a ‘friction modifier’ in other lubricant types.”
STLE-member Tyler Housel, lubricant business director for Inolex Chemical, says, “A boundary lubricity additive is similar in composition to a surfactant as it contains both polar and nonpolar groups. The polar groups are attracted to the metal (or more likely metal oxide) on the surface. The non-polar groups are often short hydrocarbon chains that are not strongly attracted to the metal oxide and form a slippery layer as they extend away from the surface. The boundary lubricity additive provides a soft, deformable layer of organic molecules that covers the hard metal oxide so the surfaces slide over each other without damaging the substrate.”
There is not a universally accepted definition of what lubricity additives are, as the functionality of these additives is mainly based on ashless, fatty chemistries. Dr. Steven Randles, global R&D director for Croda Lubricant Additives, says, “Lubricity additives might be defined as being based on organic (carbon, hydrogen, oxygen and nitrogen) chemistries that adsorb onto the metal surface. Lubricity additives tend to be based on fatty amides, fatty acids, partial esters and polymeric esters.”
Randles indicates that boundary lubricity additives are compounds or elements that react with the metal surface in a more loosely bound fashion than what is seen with EP agents. Other boundary lubricity additives that can be included are animal fatty oils (such as lard oil) and vegetable fatty oils (such as soybean oil).
A boundary lubricity additive functions through its ability to adhere to the metal surface. This property is achieved because of the presence of a polar head group contained within the structure of the additive.
Purnhagen says, “Boundary lubricity additives rely on hydrocarbon chains to serve as a fluid barrier between surfaces. Polar compounds such as synthetically prepared esters or naturally occurring triglycerides are especially effective at this function due to their ability to orient a polar head of the molecule onto a metal surface. This polarity creates a strong affinity to the metal by one end of the molecule and allows a non-polar hydrocarbon to extend out and provide a barrier between surfaces.”
Purnhagen also points out that petroleum oils, though strictly non-polar, also have some boundary lubricity functionality. But their effectiveness is limited because petroleum oils lack polar affinity to metal surfaces.
Randles offers a number of mechanisms for how boundary lubricity additives function. Most of them involve physic-sorption on the metal surface initially. He says, “Once this physi-sorption has taken place, the boundary lubricity additive can act in several different manners depending upon its structure and the operating conditions incurred by the lubricant.”
Randles believes one possible mechanism involves the breakdown of boundary lubricity additives to form fatty acids that then form metal soaps that act as antiwear/EP agents. Another possible mechanism is that higher viscosity polar polymeric compounds can be used to create thick hydrodynamic films that reduce wear. Alternatively, these thick films can be formed in situ at the metal surface via tribopolmerization.
HOW DO YOU SELECT A BOUNDARY LUBRICITY ADDITIVE?
Strong attention needs to be given to the type of application and the fluid basestock used. Purnhagen says, “Numerous considerations must be given in determining the type of boundary lubricity additive to use in a particular application. Paramount issues to review include operating temperatures and pressures, basestock solubility and fluid type.”
In metalworking fluid applications (
see Figure 1), Purnhagen maintains that boundary lubricity additive selection is critical in very high-temperature and pressure applications. Additives containing unsaturation tend to form undesirable residues and varnishes under the extreme conditions prevalent in such applications as oilbased drawing and stamping. Polymeric esters are more of a viable choice as compared to non-polymeric additives because they are able to maintain useful boundary films at higher temperatures, leading to greater degrees of friction and wear protection.
 Figure 1. Boundary lubricity additives are widely used in metalworking fluid applications. Their selection is particularly critical in high-temperature and pressure applications. (Courtesy of The Lubrizol Corp.)
Figure 1. Boundary lubricity additives are widely used in metalworking fluid applications. Their selection is particularly critical in high-temperature and pressure applications. (Courtesy of The Lubrizol Corp.)
Solubility is becoming more of a factor, particularly with the growing demand for highly refined paraffinc oils. Purnhagen says, “Many commonly used boundary lubricity additives such as esters and triglycerides have solubility limitations in Group II and Group III basestocks.”
A third factor is whether the type of lubrication fluid is oil-based or a water-based emulsion. Purnhagen says, “The emulsification of naturally occurring oils (triglycerides) is typically more challenging than a synthetically prepared ester with a more uniform structure. Self-emulsifying boundary lubricity additives, which incorporate emulsification functionality directly from an ester molecule, often are favored for emulsion systems.”
With many additive choices and applications, Housel considers selection to be a balancing act. He says, “Since operating conditions can vary widely, it is possible that any of a wide range of chemistries can be used as a boundary lubricity additive. All of these additives contain a polar group that can adsorb on the metal surface so that it becomes necessary to consider the physical form, temperature stability, compatibility with other ingredients, type of metal and other parameters when selecting a boundary lubricity additive for a specific application.”
Zhmud looks at two key rules that he indicates are based on “chemical intuition.” He says, “The first rule is for the formulator to know the type and chemistry of the additives being used. This will avoid concern regarding additive compatibility in case reformulation is required. Second, determine the tribological conditions that are typical for the application. Such criteria as the metals involved (ferrous or nonferrous), the base oil being used (petroleum or synthetics such as esters and polyalkylene glycols) and the operating conditions (load, temperature, humidity, etc.) must always be considered.”
STLE-member Toshihiro Inayama, senior technical manager for Kyowa Hakko Chemical Co. Ltd., believes evaluation of friction reduction is critical. He says, “One should choose a boundary lubricity additive that reduces friction well for the materials and temperatures expected.”
The key determining factor, according to Randles, is the application. In some cases, a second lubricant additive type may be required to work with the boundary lubricity additive. He explains, “High-load, high-temperature applications usually need a combination of antiwear and boundary lubricity additives. For milder applications, pure boundary lubricity additives can be used. But care must be taken in using acidic boundary lubricity additives because they can lead to unwanted side effects. In such cases, the formulator should select neutral additives.”
HOW DO YOU DIFFERENTIATE PERFORMANCE WITH BOUNDARY LUBRICITY ADDITIVES?
Trying to determine the best boundary lubricity additive among the many choices is challenging. STLE-member Dr. Lloyd Nelson, technology manager for Arizona Chemical Co., says, “Additive differentiation is conducted by screening potential candidates vs. benchmark materials in appropriate formulations using standard test protocols.”
Comparison of the ability of boundary lubricity additives to reduce the coefficient of friction can determine if one additive may perform better than another. For example, Inayama indicates that a pendulum type friction tester can be utilized. He says, “Under conditions of an applied load of 2.94 newtons, an initial amplitude of 0.5 radian and boundary lubricity additive concentrations of less than 0.4% in polyalphaolefin, the coefficient of friction of an amino acid-based derivative is less than an industry standard (GMO-glycerin monooleate) between 50 C and 150 C (
see Figure 2).”
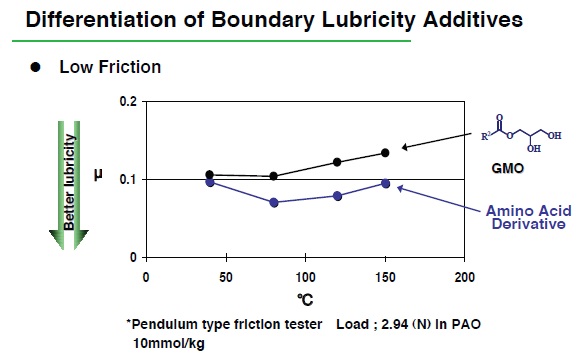 Figure 2. A pendulum-type friction tester is used to differentiate the performance between an amino acid derivative and a standard boundary lubricity additive, glycerin monooleate. The coefficient of friction for both additives changes as a function of temperature. (Courtesy of Kyowa Hakko Chemical Co., Ltd.)
Figure 2. A pendulum-type friction tester is used to differentiate the performance between an amino acid derivative and a standard boundary lubricity additive, glycerin monooleate. The coefficient of friction for both additives changes as a function of temperature. (Courtesy of Kyowa Hakko Chemical Co., Ltd.)
Purnhagen indicates that bench testing is useful as the first wave of evaluation. Test selection is dependent upon the type of applications. For example, in metalworking fluids, testing should be differentiated between metal-removal and metal-forming applications. Purnhagen says, “Metal-removal applications are better evaluated with bench methods that more closely simulate the final operation such as tapping torque. For metal-forming applications, common methods include twist compression, drawbead simulator and cup forming. Since lubricity additives are rarely the only performance additive in a finished formulation, it is important to conduct final evaluations in a complete system to properly evaluate the performance response delivered by a specific lubricity additive.”
Randles terms differentiation of one boundary lubricity additive from another as the “million-dollar challenge for the lubricant formulator.” He says, “Bench testing can be a useful predictor in some cases (e.g., lubricity additives in diesel fuel), but most lubricant applications require expensive rig tests, including gearbox. Ultimately, field trials are required.”
Zhmud maintains that measurement of lubricity is often problematic in the lab since there is no rigid definition for what the term “lubricity” stands for. He says, “Commonly used ‘lubricity’ standards such as the Ball-on-Cylinder Lubricity Evaluator (BOCLE – ASTM D 6078) and the High-Frequency Reciprocating Rig (HFRR–ASTM D 6079) underestimate the practical efficiency of boundary lubricity additives and overestimate the efficiency of EP additives. This often leads to misunderstandings, terminological muddle and endless debates regarding correlations between lab tests and field trials.”
Zhmud feels that the best way to differentiate boundary lubricity additives is through field trials and feedback from end-users. He adds, “What’s important to realize is that we never talk about the performance of an additive as such—instead, we talk about the performance of the finished product.”
HOW DO BOUNDARY LUBRICITY ADDITIVES INTERACT WITH OTHER ADDITIVES?
Various additives formulated into lubricants can compete with the metal surface. These interactions can lead to both synergistic and detrimental effects. Randles says, “Most boundary lubricity additives contain oxygen and nitrogen and are, therefore, polar. As such they interact with and compete with other surface active compounds. If correctly selected, many of these interactions can provide a positive effect.
“However, antagonisms also can occur,” Randles adds. “An incorrect boundary lubricity agent can stick to the metal surface so well as to exclude traditional antiwear chemistries and, thereby, have a marked reduction in lubricity. Poorly chosen lubricity agents can also interact with overbased detergent systems and cause clouding by being so surface active they disrupt the alkylbenzene on the calcite surface.”
Zhmud points out that additives incorporated into a specific formulation can either have synergistic effects, antagonistic effects or no effect whatsoever. He says, “What is essential to know for the formulator is that effects of additives do not really add. There are three types of incompatibilities: (1.) a solvency shift, which may destabilize the formulation or affect adsorptivity of other additives; (2.) competitive adsorption, which may undermine efficiency of some surfacereactive EP additives; and (3.) chemical reactions that may inhibit some additives or produce unwanted reaction products.”
The one additive class that most respondents consider to interact with boundary lubricity additives is the EP agent. A better understanding of the interaction of boundary lubricity with EP additives should enable the formulator to better use both additives.
HOW DO YOU DIFFERENTIATE BETWEEN BOUNDARY LUBRICITY AND EP ADDITIVES?
Nelson says, “Boundary lubricity additives are recommended for applications where metal-to-metal contact is to be mitigated under low temperature and load conditions.”
With both boundary lubricity and EP agents operating under boundary lubricant conditions, it is best to review the Stribeck Curve (
see Figure 3), a plot of the coefficient of friction (on the vertical axis) vs. a term (on the horizontal axis) that is proportional to the viscosity and relative speed of the surfaces and inversely proportional to the load. In moving from right to left, if the viscosity or speed decrease or the load increases, then the friction climbs because two surfaces move closer to each other in transitioning from the film to the boundary lubrication regimes.
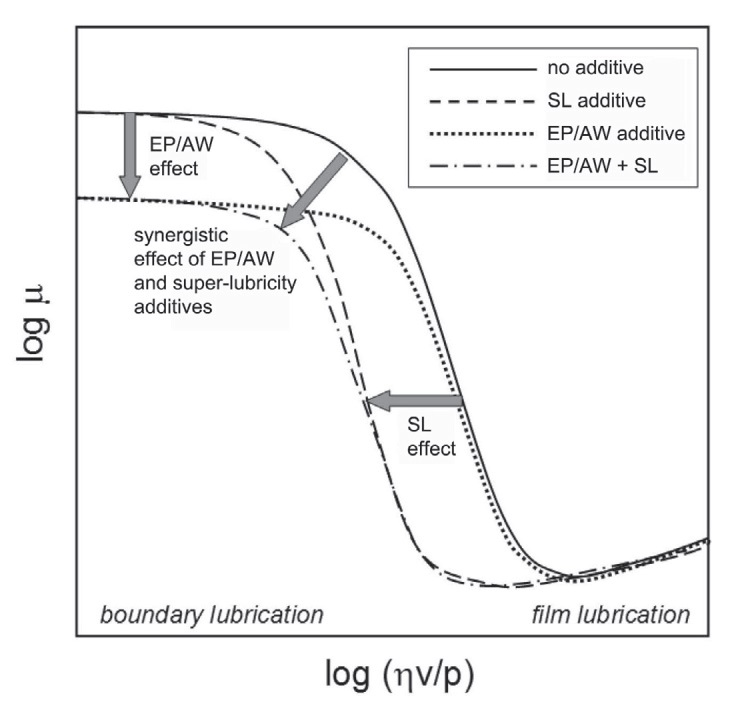 Figure 3. The Stribeck Curve shows the benefits of super-lubricity additives as the lubrication moves from a film to a boundary regime. Super-lubricity additives generate viscoelastic surface layers expanding the range of operating conditions under which film lubrication is sustained. (Courtesy of E-ION Additives)
Figure 3. The Stribeck Curve shows the benefits of super-lubricity additives as the lubrication moves from a film to a boundary regime. Super-lubricity additives generate viscoelastic surface layers expanding the range of operating conditions under which film lubrication is sustained. (Courtesy of E-ION Additives)
Zhmud indicates that boundary lubricity additives differ from extreme pressure agents in how they reduce friction and wear on the metal surface. He says, “A boundary lubricity additive forms an adsorbed surface layer or slippery surface deposits while an EP additive reacts with the metal surface to yield a reaction product that renders the surface more slippery.”
Factored into the additive interactions is the function of what Zhmud considers a special type of boundary lubricity additive that is designated as a super-gel-forming lubricity additive or super-lubricity additive (SL in Figure 3). He says, "Super-lubricity additives form a sponge-like viscoelastic surface layer retaining the base oil in the tribocontact even at zero sliding speed, thus expanding the range of operating conditions under which film lubrication is sustained."
Zhmud considers super-lubricity additives to build upon the concept of biomimetic lubrication that nature has perfected in applications such as human joints. Zhmud adds, "One example is the algae slime that grows on rocks at the seashore. Algae retain a sufficiently thick layer of water that acts as a lubricant, making the rock slippery."
As shown in Figure 3, super-lubricity additives provide added benefit (SL effect) by retaining the base lubricant between rubbing surfaces, thus expanding the range of operating conditions under which film lubrication is sustained. They also work synergistically with extreme pressure agents to further reduce friction at higher loads when the lubricant film breaks down.
Randles indicates that viscous polymeric esters act in the fashion of super-lubricity additives in combination with antiwear additives. He says, "Polymeric ester lubricity agents stick to the metal surface and create a thick protective film that provides protection to zinc dialkyldithiophosphate (ZDDP) antiwear additives. In addition, the oxygen within the ester can contribute to the formation of a hard polyphosphate ZDDP layer. If such interactions are optimized, it allows for a significant (e.g., 50%) reduction in the amount of ZDDP required to deliver wear performance."
Polarity of the polymeric ester is very important to ensure that an effective film is produced with the ZDDP. Randles explains, "If the polymeric ester is too polar, it may interfere with the formation of the hard polyphosphate film and result in an increase in wear. It is therefore important to ensure that the polarity of the lubricity agent is optimized to sit on top of the ZDDP rather than underneath it
(see Figure 4)."
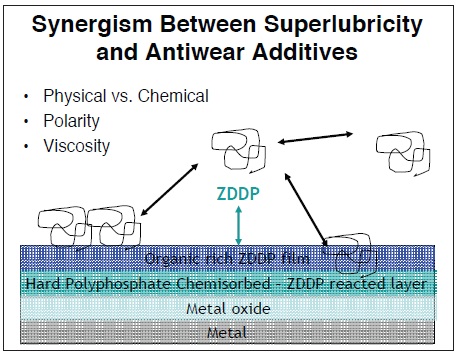 Figure 4. Super-lubricity additives can form a thick protective film over a ZDDP-based antiwear layer to improve performance. If the interaction between the two additives is optimized, a significant reduction in the amount of ZDDP used can be realized (Courtesy of Croda Lubricants Additives)
Figure 4. Super-lubricity additives can form a thick protective film over a ZDDP-based antiwear layer to improve performance. If the interaction between the two additives is optimized, a significant reduction in the amount of ZDDP used can be realized (Courtesy of Croda Lubricants Additives)
Randles asserts that when employed properly, boundary lubricity additives can enable the formulator to significantly reduce the concentration of EP agents used in a specific application by more than 50%.
Boundary lubricity additives are used with EP agents to cover the entire temperature range of applications, as shown in Figure 5. Purnhagen indicates that this approach is very important in oil-based lubricant formulations. He adds, "Certain light duty oil-based applications such as copper alloy machining often can be accomplished with a boundary lubricity additive alone."
 Figure 5. Boundary lubricity additives can be used with extreme pressure additives to cover the entire temperature range of applications. This approach is particularly important in oil-based lubricant formulations. (Courtesy of The Lubrizol Corp.)
Figure 5. Boundary lubricity additives can be used with extreme pressure additives to cover the entire temperature range of applications. This approach is particularly important in oil-based lubricant formulations. (Courtesy of The Lubrizol Corp.)
For water-based applications such as metalworking, temperatures do not always rise high enough to activate EP agents. Purnhagen says, "In water-based systems, many applications receive adequate performance from a lubricity additive alone, such as high-speed machining on softer metals like aluminum. This can be attributed to the high cooling capacity of water-based fluids."
Use of EP additives, in conjunction with boundary lubricity additives, should only be done if the operating conditions warrant. Housel says, “Sometimes EP additives can have detrimental effects on the lubricant. They are chemically active and can increase oxidation, causing deposits and sludge to form at higher temperature. Unless EP conditions are expected, I would not add EP additives to a lubricant.”
Zhmud picks up on this point by stating that there exists an erroneous belief that EP additives never harm because (1.) they are needed in case the extreme conditions associated with intense wear are encountered and (2.) there is not much friction and wear to worry about otherwise. He adds, “Many tribosystems that operate for a lifetime under hydrodynamic or elastohydrodynamic conditions still suffer from wear. An example is the wire/die system in aluminum wet wiredrawing. In this case, wear occurs because of hard impurities accumulating in the lubricant. Boundary lubricity additives delocalize stresses, solubilize particulate and reduce wear under these conditions. In contrast, EP additives are not activated and can cause corrosion and pitting wear of dies. Another example is gear lubricants in which some EP additives deployed to control scuffing may cause micropitting.”
For this reason, Zhmud recommends boundary lubricity additives in applications where film strength is more important.
WHAT IS METATHESIS CHEMISTRY?
Most boundary lubricity additives are prepared by the synthetic modification of naturally derived fatty oils. Petrochemicals also can be used, but the trend toward development of sustainable or green technologies means that this approach is not as favored.
Metathesis is a well-known reaction and has been used to manufacture conventional petrochemicals. Andy Shafer, executive vice president of sales and market development for Elevance Renewable Sciences, Inc., says, “Metathesis is a word meaning ‘changing places.’ In olefin metathesis, groups bonded to the double bond change places with one another.”
One petrochemical example is the conversion of butylene and ethylene to two molecules of propylene. However, the catalyst used in this type of metathesis does not work with compounds with polar functional groups such as in naturally derived fatty oils. The metathesis reaction of such compounds is facilitated by metal carbene catalysts. With the invention of these metal carbene catalysts, metathesis is now being applied to transform naturally derived fatty oils into products that may be used in the future to provide boundary lubricity.
A mechanism for olefin metathesis proposed by Yves Chauvin can be viewed as a dance in which the “catalyst pair” and the “olefin pair” dance around and change partners with one another (4). The metal and its partner hold hands with both hands and when they meet the olefin pair the two pairs unite in a ring dance. After a while they let go of each other’s hands, leave their old partners and dance on with their new ones. The new catalyst pair is now ready to catch another dancing olefin pair for a new ring dance or, in other words, to continue acting as a catalyst in metathesis. This reaction is shown in Figure 6.
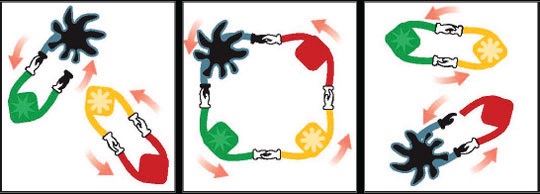 Figure 6. The mechanism for the metathesis reaction is analogous to a dance between a catalyst pair and an olefin pair. Part way through the process, there is a change in partners. Traditionally, this reaction has just been used with petrochemicals but now it has been adapted for use in molecules with polar functional groups. (Courtesy of Elevance Renewable Sciences)
Figure 6. The mechanism for the metathesis reaction is analogous to a dance between a catalyst pair and an olefin pair. Part way through the process, there is a change in partners. Traditionally, this reaction has just been used with petrochemicals but now it has been adapted for use in molecules with polar functional groups. (Courtesy of Elevance Renewable Sciences)
Shafer says, “We have applied the metathesis catalysts developed by Nobel Prize winner Dr. Robert Grubbs to conduct the metathesis reaction on molecules that contain polar functional groups. This, in effect, enables us to convert natural feedstocks by metathesis into novel products that may have application as boundary lubricity additives.”
Potential feedstocks include: algae, canola, jatropha, palm and soybean oils. Shafer adds, “We can also work with any molecule that has a double bond, including unsaturated fatty acids and their corresponding esters.”
The metathesis approach enables the creation of longer or shorter carbon chains to create oligomers and polymers. In addition, other functional groups can be inserted or exchanged. Shafer says, “This flexibility enables us to develop products that can be very difficult or impossible to synthesize using petrochemical routes.”
From a lubricant standpoint, properties that can be improved upon are thermal/oxidative stability and pour points. Shafer says, “We envision that our ability to move the olefin group can lead to the development of additives that are more stable over wider temperature ranges.”
At this point, Shafer indicates that work is just starting on developing products for the lubricant market using metathesis.
WHAT FUTURE TRENDS CAN WE EXPECT WITH BOUNDARY LUBRICITY ADDITIVES?
Several respondents indicate that development of multifunctional boundary lubricity additives will be a very significant factor in providing formulators with technologies that provide more effective performance.
Randles says, “One of the main disadvantages of boundary lubricity additives is their potential to interact with other additives in the formulation. By making multifunctional lubricity agents, some of these disadvantages can be removed. For example, incorporating emulsification and lubricity into the same molecule can lead to significant improvements in wear performance, especially with hard-to-machine lighter-weight alloys.”
The organic nature of lubricity additives allows them to be made from renewable raw materials, an obvious advantage in ecologically sensitive applications. Their ability to act synergistically with traditional antiwear chemistries (thereby reducing their dose rates) allows the use of lubricity additives in low sulfated ash and phosphorous formulations. As these are key market drivers, further developments in these areas are anticipated in the future.
Purnhagen sees the potential particularly in the preparation of water-emulsifiable fluids. He says, “Wider application of self-emulsifiable ester technologies that combine boundary lubricity with emulsification eliminates the challenges of achieving stable emulsions of non-aqueous esters and triglycerides.”
Purnhagen also believes that there will be wider use of emulsifiers, which will contribute supplemental boundary lubricity and reduce the need for total reliance on one additive in applications such as metalworking fluids.
Inayama agrees that additives that can provide multifunctional benefits, including boundary lubricity, will be more readily available in the future. He says, “These additives also will be derived from new natural sources such as amino acids. They will have the capability to reduce friction and wear on metal surfaces under less severe boundary lubrication conditions prior to the activation of EP additives.”
Zhmud indicates that boundary lubricity additives have the properties to enable development of energy-efficient lubricants. He says, “The change to lower-viscosity oils has been accelerating due to the need to improve the energy efficiency of lubricants. It should be realized, however, that the energy savings are often achieved not by reducing friction, as many are keen to believe but, rather, by reducing viscous bulk losses due to changing to lower-viscosity oils. Boundary lubricity additives allow the formulator to use thinner oils without increasing the risk of wear. The most promising in this respect are superlubricity additives.”
One other issue is the trend toward Group II through Group IV base oils, which leaves the need for finding added lubricity. Zhmud explains, “Group I base oils have sufficiently high content of polar species (heterocycles, aromatics) and demonstrate superior lubricity as compared to Group II-IV base oils that contain predominantly fully saturated nonpolar hydrocarbon chains. Thus, greater use of boundary lubricity additives will be helpful to boost performance.”
Housel foresees greater demand for boundary lubricity additives. He says, “Reliance on boundary lubricity additives will increase as more attention is paid to improving the efficiency of machinery by reducing friction and wear that can occur as two surfaces interact with each other.”
Boundary lubricity additives were the first type of lubricants used in the mid-1800s. The lubricant industry appears to have gone full circle and is placing a greater emphasis now on using these additives to improve the effectiveness of lubricants now and in the future.
REFERENCES
1.
Canter, N. (2007), “Special Report: Trends in Extreme Pressure Additives,” TLT,
63 (9), pp. 10–18.
2.
Hermann, C. and McGlade, J. (1974), “Industrial Applications for Animal Fatty Oils,” Journal of the American Oil Chemists’
Society,
51 (3), pp. 88–92.
3.
De Guzman, D. (2009), “Back to the Lab,”
ICIS Chemical Business,
275 (20), pp. 30–32.
4.
Chauvin, Y. and Herisson, J.-L. (1971), “Catalyse De Transformation Des Olefins Par Les Complexes Du Tungstene. II. Telomerisation Des Olefins Cycliques En Presence d’Olefines Acycliques,”
Makromolekulare Chemie,
141, pp. 161–176.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.