Scrambling oxygen
Dr. Neil Canter, Contributing Editor | TLT Tech Beat July 2009
A five-step process illustrates the interaction of an oxygen adatom and water on the surface of titanium dioxide.
KEY CONCEPTS
•
Titanium dioxide is a photosensitizer that can be used as a catalyst to generate hydrogen from water.
•
Evaluation of the interaction of titanium dioxide with water in the presence of oxygen shows that oxygen adatoms appear to be jumping over the oxygen rows of titanium dioxide.
•
The water molecules appear to catalyze the movement of oxygen adatoms, which, in turn, facilitate the dissociation of water into hydrogen and oxygen.
Titanium dioxide is a pigment that is commonly used in sunscreens to help protect against UV radiation when our skin is exposed to the sun. This compound is very useful in sunscreens because it resists discoloration when exposed to UV light.
Titanium dioxide also is being evaluated as a photosensitizer that can catalytically split water into oxygen and hydrogen. This property means that titanium dioxide has potential for use in fuel cells.
Dr. Igor Lyubinetsky, physicist at the Department of Energy’s Pacific Northwest National Laboratory, says, “We have been working with titanium dioxide as part of a Hydrogen Fuel Initiative for the Department of Energy. Research in this area has provided a good understanding of the interactions between water and titanium dioxide.”
An area of interest that has implications, not just for hydrogen fuel production, but also for potentially providing further insight into the catalytic mechanism, is the activity of titanium dioxide and water in the presence of oxygen.
The surface of the titanium dioxide has the appearance of a corn field with oxygen atoms in rows that rise away from the titanium atoms. During the reduction process, single oxygen atoms from the rows are lost, which opens up vacancies. These vacancies can be controlled by the temperature and heating duration of titanium dioxide.
These sites are very reactive. Molecular oxygen (O
2) coming into contact with a vacancy will split. One of the oxygen atoms fills the vacancy and the other becomes an adatom on the surface.
Lyubinetsky says, “Exposure of oxygen to titanium dioxide readily leads to the placement of oxygen atoms on titanium atoms. These oxygen atoms are known as adatoms, which is an abbreviation for adsorbate atom. Reduction of the titanium dioxide surface through the use of heat in a vacuum leads to the removal of these adatoms.”
Analysis of the reaction of water with oxygen in the presence of titanium dioxide may provide further indication of how the catalytic process can spread across a metal surface.
DANCING ATOMS
Lyubinetsky and his coworkers used scanning tunneling microscopy (STM) and density functional theory to study the interaction of water with oxygen and titanium dioxide. Experiments using STM were conducted at 300 K in a vacuum system with a pressure less than 10
-10 torr.
The researchers initially introduced oxygen adatoms to the titanium dioxide. Water was then added and STM was used to study the motion of the oxygen adatoms.
Lyubinetsky says, "We found that the oxygen adatoms appeared to jump among the titanium atoms. This action occurs not only along the row of titanium atoms but also across different rows of titanium atoms. It appears as if the oxygen adatoms are jumping over the rows of titanium atoms."
STM analysis also determines that a pair of hydroxyl groups is formed during this process. Lyubinetsky explains, "We believe that water molecules catalyze the movement of oxygen adatoms from one titanium atom to another. This phenomenon gives the appearance of oxygen atoms dancing among the titanium atoms. In actuality, the data shows that the oxygen atoms are scrambling and not really moving."
The process can be described by the reversible reaction scheme shown in the following equation:
H
2O + O ↔ 2 OH
Figure 3 divides the reaction into five steps taken to generate the two hydroxyl group pairs from an oxygen adatom and water. In this diagram, oxygen atoms are shown in red, hydrogen atoms are in blue and titanium atoms are in white.
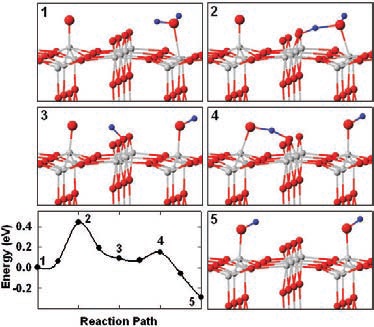 Figure 3. The interaction of an oxygen adatom and water on the surface of titanium dioxide is illustrated as a five-step process. Oxygen atoms are shown in red, hydrogen atoms are in blue and titanium atoms are in white. (Courtesy of the Department of Energy’s Pacific Northwest National Laboratory)
Figure 3. The interaction of an oxygen adatom and water on the surface of titanium dioxide is illustrated as a five-step process. Oxygen atoms are shown in red, hydrogen atoms are in blue and titanium atoms are in white. (Courtesy of the Department of Energy’s Pacific Northwest National Laboratory)
Frame 1 shows the positioning of a water molecule next to oxygen adatoms. A hydrogen atom is stolen from the water molecule by an oxygen adatom to form two hydroxyl groups in Frame 2. One of the hydroxyl groups swings around in Frame 3 so that the hydrogen atom can be stolen by a third oxygen atom in Frame 4. This leads to the apparent movement of one of the hydroxyl pairs to a different position along the row of titanium atoms shown in Frame 5.
In effect oxygen adatoms facilitate the dissociation of water. In turn, water molecules appear to catalyze the movement of the oxygen adatoms and are not consumed in the process.
Frame 5 shows that the energy barrier for the forward reaction is lower than for the reverse process back to water and the oxygen adatom. Lyubinetsky adds, "We attribute the high barrier of the reverse reaction to be due to hydrogen bonding preventing water from diffusing away from the oxygen adatom."
Future work will focus on gaining a better understanding of this process by varying the concentration of oxygen adatoms and temperature. STM cannot see the movement of the water molecules at 300 K because they move too fast under these conditions. Lyubinetsky says, "We will drop the reaction temperature to see evidence for the presence of the water molecules acting to catalyze the process."
The findings described here are of a fundamental nature and provide clues about how water can be split into hydrogen and oxygen. The water is present in the environment at a low concentration in the vapor state. Scientists also would like to raise the temperature of the reaction by 50 to 100 K to see how much faster the oxygen adatoms can move themselves.
From the photocatalysis standpoint, Lyubinetsky considers metal clusters placed on the surface of titanium dioxide to be a system that can be studied to determine further information about the mechanism. Palladium is an example of a metal catalyst that facilitates the splitting of water by titanium dioxide in the presence of light.
Further information can be found in a recently published article (
1).
REFERENCE
1.
Du, Y., Deskins, N., Zhang, Z., Dohnalek, Z., Dupuis, M. and Lyubinetsky, I. (2009), "Two Pathways for Water Interaction with Oxygen Adatoms on TiO
2 (110),"
Physical Review Letters,
102 (9), 096102.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.