Boron-based ionic compounds
Dr. Neil Canter, Contributing Editor | TLT Tech Beat May 2009
The formation of boron boride is contrary to the classic chemistry that we all learned in high school and college.
KEY CONCEPTS
•
Boron compounds are used as solid lubricants in a number of applications.
•
A new ionic compound has been discovered that is solely based on boron.
•
The existence of this boron boride is contrary to classic chemistry that indicates ionic bonds are only formed between elements of different electronegativities.
When discussed, the term “ionic compound” conjures images of an inorganic solid that contains a metal cation and a nonmetallic anion in a crystalline lattice structure. A compound such as sodium chloride comes to mind in which the sodium and chlorine atoms are arranged in a close-packed cubic crystal structure.
There are a myriad of other ionic compounds based on other inorganic elements. Most of these compounds form compact lattices with high melting points. For example, the melting point of sodium chloride is approximately 800 C.
Recently, a different class of ionic compounds has been developed that are liquids at room temperature and have melting points no higher than 100 C. Ionic liquids are formed by combining bulky asymmetric anions and cations. Many of these are based on organic compounds. Over the past 20 years, approximately one million ionic liquids have been developed. In a previous TLT article, ionic liquids based on polyethers and fluorinated species were being evaluated as high temperature lubricants (
1).
Boron compounds have been used as solid lubricants in a number of applications. For example, Boron nitride is used as a solid lubricant, particularly in high temperature applications which take advantage of the material’s good lubricity and thermal conductivity.
Boric acid is another example of a solid lubricant. In a recent TLT article, evaluation of nano-sized boric acids shows better dispersibility in mineral oil basestocks, which translates into a more effective reduction of the coefficient of friction of liquid lubricants (
2). This has the potential to be used in applications such as automotive lubricants.
Boric acid forms a crystalline lattice that is held together by a combination of ionic bonding, covalent bonding and hydrogen bonding. The bonding enables nano-boric acid to form surface boundary film in which the layers in the lattice can slide easily over one another.
Boron is an electron-deficient element that has relied on complex structures to bond a series of boron atoms and satisfy the octet rule. In the case of clusters with 12-boron atoms, the main structure formed is an icosahedron.
Artem Oganov, associate professor in the department of geosciences and New York Center for Computational Science at the State University of New York at Stony Brook, says, “Boron is a frustrated element. In the periodic table, it is located right between metals and insulators; this element does not know what to do and ends up having extremely complex chemistry.”
New phases of 12-boron atom clusters can be generated under conditions of higher temperatures and pressures. The objective of producing these phases is to find a form of boron that is the true ground state and displays greater stability than the others.
BORON BORIDE - B2B12
Oganov and a group of researchers have synthesized a new boron atom cluster that has different properties than those previously discovered. The cluster is formed by heating a boron phase known as beta-B
106 (crystalline structure with 106 atoms in the unit cell) at a temperature in excess of 2,450 K at above 10 gigapascals (GPa) of pressure.
Oganov says, “The resulting product is a crystalline solid that is also quenchable to ambient temperature and pressure. It can be held in one’s hand without any problems.”
Oganov used a combination of experimental data and a new theoretical method to determine the structure of the product. He says, “We determined that a single phase of boron is formed that has 28 atoms in a unit cell. The atoms are organized into two different types of nanoclusters, which contain a 12-boron atom cluster forming an icosahedron and a two-atom linear boron structure.”
Figure 3 shows the crystal structure of this new phase of boron. The 12-boron atom cluster is shown in purple, and the two-boron atoms are illustrated in orange and have the appearance of dumbbells.
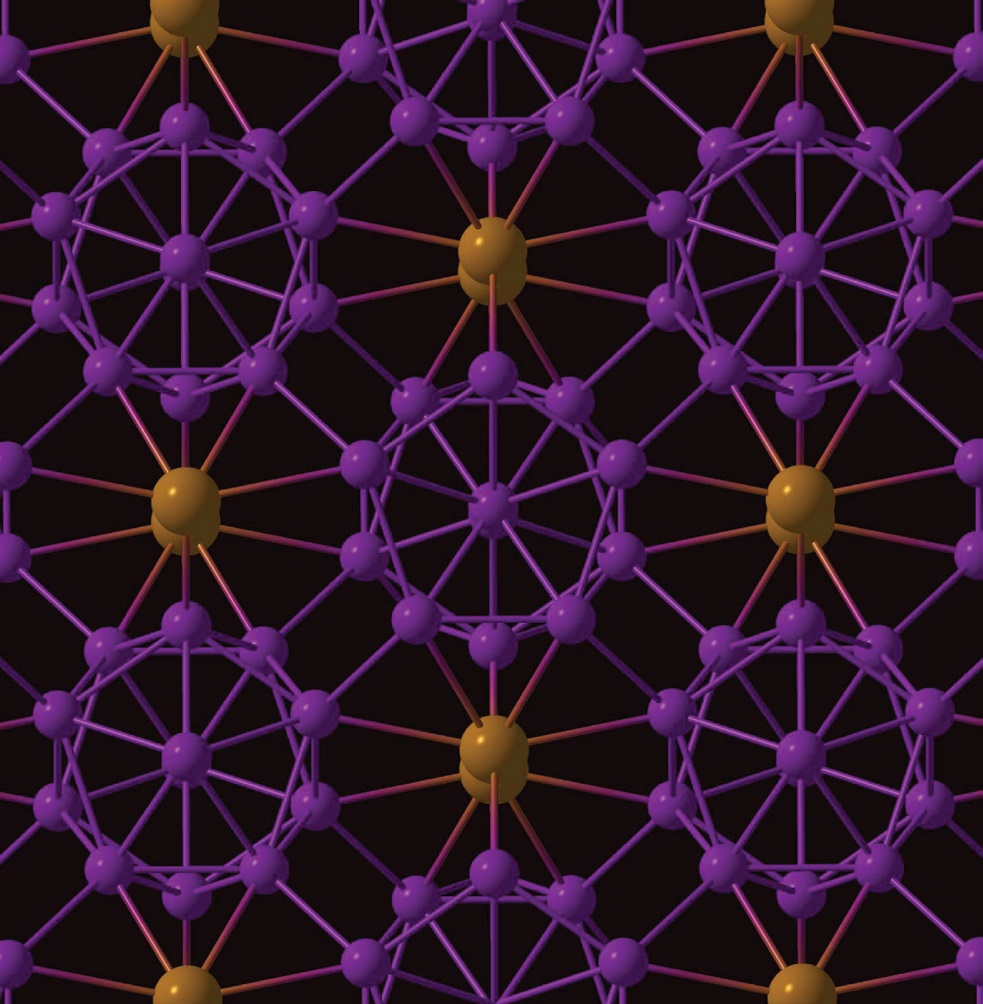 Figure 3. A new ionic compound solely based on one element, boron, has been prepared. The crystal structure consists of a 12-boron atom cluster shown in purple and two-boron atoms shown in orange. (Courtesy of the State University of New York at Stony Brook)
Figure 3. A new ionic compound solely based on one element, boron, has been prepared. The crystal structure consists of a 12-boron atom cluster shown in purple and two-boron atoms shown in orange. (Courtesy of the State University of New York at Stony Brook)
Oganov says, “Significant charge transfer was found between the B
2 and B
12 clusters. We found that the B
2 pairs within the lattice display a slight positive charge, while the B
12 icosahedron has a slight negative charge. This means that this new phase of boron can be considered a boron boride with the molecular formula B
2B
12.”
The crystalline structure of the boron boride is very similar to that of sodium chloride, according to Oganov. He adds, “The centers of the B
12 icosahedra form a slightly distorted cubic close-packing structure. Voids in the octahedral structure are occupied by the B
2 pairs.”
Oganov considers boron boride to be a different form of boron in an analogous fashion to the relationship between diamond and graphite. He says, “Boron boride is very similar in features to diamond. Both materials are less stable than other forms of their corresponding elements and can only be formed under extreme conditions. But once formed, both materials are very stable.”
Oganov notes that this boron boride is still composed predominantly of covalent bonds, but the degree of iconicity is substantial. The ionic characteristics seen from the charge transfer analysis also are reflected in the large difference between high-frequency and static dielectric constants and in infrared absorption.
The formation of boron boride is contrary to the classic chemistry that we all learned in high school and college. Ionic bonds are supposed to be formed between elements of different electro-negativities.
The potential use for boron boride as a lubricant is unknown. But boron compounds have been shown to display outstanding lubricant characteristics. It may be worthwhile to explore the lubricating properties of this interesting material in the future.
Oganov also believes that other elements will be found to display significant ionic characteristics. Future work will be conducted to prepare an ionic version of carbon from a fullerene crystal.
Further information on this research can be found in a recent article (
3).
REFERENCES
1.
Canter, N. (2007), “Using Dicationic Liquids as High Temperature Lubricants,” TLT,
63 (5), pp. 12–13.
2.
Canter, N. (2008), “Friction-Reducing Characteristics of Nano-Boric Acid,” TLT,
64 (2), pp. 10–11.
3.
Oganov, A., Chen, J., Gatti, C., Ma, Y., Ma. Y., Glass, C., Liu, Z., Yu, T., Kurakevych, O. and Solozhenko. V. (2009), “Ionic High-Pressure Form of Elemental Boron,”
Nature,
457 (7231), pp. 863–867.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.