Magnetic refrigeration: Another way to cool
Dr. Neil Canter, Contributing Editor | TLT Tech Beat May 2009
This emerging technology consists of a safer, more cost-effective alloy based on manganese, iron, phosphorous and germanium.
KEY CONCEPTS
•
Magnetic refrigeration is an alternative approach to cooling that does not require a refrigerant.
•
This process uses a metal alloy called a magnetocaloric that heats up when exposed to a magnetic field and cools off when the magnetic field is removed.
•
A new magnetocaloric alloy has been developed based on manganese, iron, phosphorous and germanium that is very attractive because it generates a large magnetocaloric effect at room temperature.
The traditional technology used in refrigeration is gas compression, which we widely use in home refrigeration and air conditioners. Four key elements present in the traditional refrigeration cycle are the evaporator, compressor, condenser and expansion valve, as shown in Figure 1.
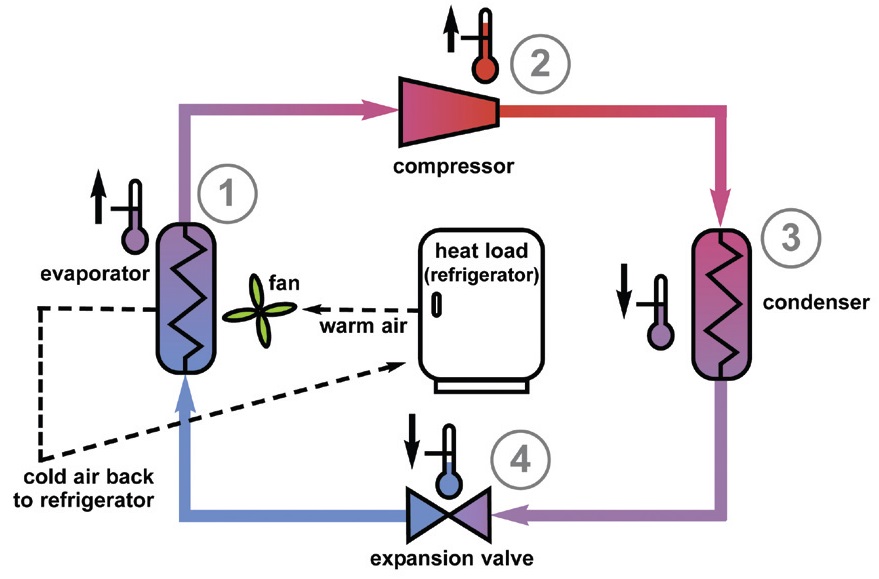 Figure 1. The traditional refrigeration cycle requires the use of an evaporator, compressor, condenser and expansion valve. (Courtesy of the National Institute of Standards and Technology (NIST))
Figure 1. The traditional refrigeration cycle requires the use of an evaporator, compressor, condenser and expansion valve. (Courtesy of the National Institute of Standards and Technology (NIST))
The process initially starts with liquid refrigerant under low pressure coming from the expansion valve (Step 1). This liquid then passes through the evaporator during which it picks up heat from the environment, vaporizes and, as a result, sends cold air back to the system. The resulting low pressure refrigerant gas coming from the evaporator is compressed into a high pressure gas at a higher temperature by the compressor (Step 2). The gas moves through a condenser (cooling coils) which leads to its condensation into a high pressure liquid (Step 3). At this stage, the heat is removed from the refrigerant. The liquid passes through an expansion valve that reduces its pressure and, as a consequence, its boiling point (Step 4).
The refrigeration lubricant plays a critical role in minimizing compressor wear, preventing corrosion and removing heat efficiently during the compression process. In a previous TLT article, studies reviewing the use of copper (II) oxide nanoparticles in a conventional polyol ester-based refrigeration lubricant and the refrigerant R134a showed that the presence of these nanoparticles can boost heat transfer performance (
1).
Dr. Jeffrey Lynn, fellow and team leader, Condensed Matter Physics at the National Institute of Standards and Technology (NIST), says, “Conventional gas compression technology has its drawbacks. This is due to the use of HCFC (hydrochlorofluorocarbon) refrigerants that can have a negative impact on the environment. One other consideration is that it has become more difficult to improve this refrigeration process. The efficiency of the gas cycle has pretty much maxed out.”
One alternative approach that has been evaluated is to utilize a magnetic field to conduct the cooling. Magnetic refrigeration has been studied in the past but has mainly relied on alloys with two elements, gadolinium and arsenic, that have significant drawbacks. The former is very expensive, and the latter is a deadly toxin.
An alternative alloy that is safer and more cost-effective has not been developed until now.
EXOTIC METAL ALLOY
An international research team working at the NIST’s Center for Neutron Research (NCNR) has developed an exotic metal alloy based on manganese, iron, phosphorus and germanium that may provide the impetus to enable magnetic refrigeration to be commercialized for everyday uses.
Alloys used in this type of refrigeration are called magnetocalorics because they heat up when exposed to a powerful magnetic field and then cool off when the magnetic field is removed.
In magnetic refrigeration (
see Figure 2), a magnetocaloric absorbs heat expelled from a refrigerator (Step A). When exposed to an electromagnetic field, the magnetocaloric becomes aligned and heats up (Step B). The magnetocaloric can then expel heat while remaining in the electromagnetic field (Step C). This leads to a reduction in temperature. Once the field is turned off, the temperature of the magnetocaloric drops more significantly, leading to material being left in the disordered state (Step D) and ready to go through the cycle again by absorbing heat from the refrigerator.
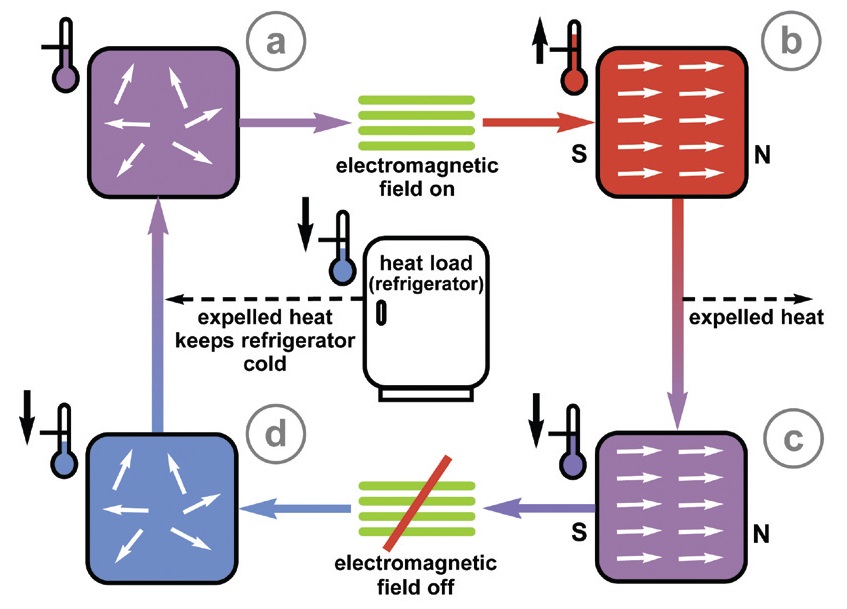 Figure 2. Magnetic refrigeration uses a metal alloy instead of a refrigerant to absorb and then expel heat in the presence of a magnetic field. No refrigerant is needed but a heat transfer mechanism utilizing a lubricant will be required. (Courtesy of the National Institute of Standards and Technology (NIST))
Figure 2. Magnetic refrigeration uses a metal alloy instead of a refrigerant to absorb and then expel heat in the presence of a magnetic field. No refrigerant is needed but a heat transfer mechanism utilizing a lubricant will be required. (Courtesy of the National Institute of Standards and Technology (NIST))
The key to the process is that the magnetocaloric moves from a disordered, paramagnetic state to an ordered, ferromagnetic state. In the latter, the electron spins in the magnetocaloric line-up.
The transition process from a disordered to an ordered state in the presence of a magnetic field can be a first-order transition comparable to ice melting. Lynn says, “During the melting of solid ice to water, the temperature does not change. A latent heat figure is obtained, which indicates the amount of heat absorbed in the process. In an analogous fashion, the magnetocaloric effect details how much of a temperature change is generated by using the magnetic field to drive the transition from a disordered to an ordered state.”
He adds, “The movement from an ordered magnetocaloric aligned in the field to a disordered state is called adiabatic demagnetization.”
The important factor is to find magnetocalorics that will conduct this process near room temperature. Iron undergoes this transition at a temperature of 1,000 K, according to Lynn.
Rare earth metals have very large magnetic moments and are likely candidates for use in magnetic refrigeration such as gadolinium, which has a room temperature transition. However, it is too expensive to be commercially viable and also it does not have a sufficiently good magnetocaloric effect (5 joules per kilogram Kelvin) to be acceptable for this application.
The new alloy, based on manganese, iron, phosphorus and germanium, displays a magnetocaloric effect at room temperature of 74 to 78 joules per kilogram Kelvin, which is the highest reported so far for any material. In addition, the cost of this alloy is much lower than gadolinium.
Lynn indicates that future work will involve improving the properties of the alloy so that it can be used commercially. He says, “We need to be able to see a large magnetocaloric effect with a smaller magnetic field. Currently, the 5 Tesla field being used is too big. We need to reduce the field strength to 2 Tesla.”
The properties of the alloy also need to be improved in order to boost the magnetocaloric effect. Lynn does not see any durability problems with the magnetic material. He envisions that a disc of the magnetocaloric material can be used in a refrigerator with only a portion being exposed at any one time to the magnetic field.
The magnetic refrigeration process also could be used to run a heat pump. The big difference is that no refrigerant is needed, which eliminates the possibility of emissions.
Lynn emphasizes that the large amount of heat generated when the magnetocaloric material becomes paramagnetic means that a conventional pumped cooling system with an associated lubricant will still be required to act as a heat transfer mechanism in a similar fashion to the current gas compression technology.
Lynn says, “There is also no reason why this magnetic refrigeration technology could not be used in mobile applications such as automobiles.”
Further information can be found in a recent reference (
2) or by contacting Lynn at
jlynn@nist.gov.
REFERENCES
1.
Canter, N. (2008), “Improving Refrigeration with Nanoparticles,” TLT,
64 (11), pp. 14–15.
2.
Liu, D., Yue, M., Zhang, J., McQueen, T., Lynn, J., Wang, X., Chen, Y., Li, J., Cava, R., Liu, X., Altounian, Z. and Huang, Q. (2009), “Origin and Tuning of the Magnetocaloric Effect in the Magnetic Refrigerant Mn
1.1Fe
0.9(P
0.8Ge
0.2),”
Physical Review,
B 79 (1), 014435.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.