Crack formation in brittle materials
Dr. Neil Canter, Contributing Editor | TLT Tech Beat February 2009
Researchers use computer modeling and experimentation to study the movement of cracks at low speeds through silicon.
KEY CONCEPTS
•
Cracks moving at slow speeds move in different orientations than at higher speeds and can affect the physical properties of the material on a macroscopic level.
•
A crack moving through a silicon crystal at a slow speed generates triangular ridges that deviate in the same direction.
•
As the crack breaks through six-member rings of silicon atoms, reconstruction of the area disrupted occurs through the formation of five-member and seven-member rings.
Brittle materials such as glass and stone tend to fracture when pressure is applied. The failure mechanism for this fracture involves the initiation and propagation of a crack.
The fracturing of brittle materials is an important issue that needs to be overcome in such applications as tough coatings, micromachines and armor. Dr. Noam Bernstein, research physicist with the Naval Research Laboratory in Washington, D.C., says, “Flaws or cracks are inherently present in brittle materials. The surface of a brittle material may appear to be smooth from the macroscopic standpoint, but there are microscopic cracks and little voids between grains. If enough stress is placed on a material, the longest preexisting crack can move across and produce a catastrophic failure.”
The speed at which a crack moves through a brittle material affects how it propagates, according to Bernstein. He adds, “Past analysis using continuum mechanics theory shows that a crack will not start to move through a brittle material until a certain load is reached. Experimentally, we know that cracks at moderate speed will move forward through the material in a smooth way fashioning a straight line. At high-speeds, cracks tend to oscillate through the material, creating rough surfaces.”
Movement of cracks at low speeds is not well understood but important in understanding how brittle materials can fail. This phenomenon could not be studied until now because computer simulation and experimental results were not available.
EVALUATION IN SILICON
Bernstein and a group of researchers used computer modeling and experimentation to study the movement of cracks at low speeds through silicon. He says, “We chose to use single crystalline silicon in our simulations and pure silicon wafers in the experimental studies. Silicon is available in a very pure state and has been studied extensively because of its use in the electronics industry.”
Cracks moving at high-speeds through silicon travel in the range of 1 to 2 kilometers per second. In contrast, slow speed crack movement occurs at a rate of 100 to 200 meters per second.
Computer modeling using a technique called “Learn on-the-fly” provides a better understanding of how cracks move at low speeds. This approach enables the researchers to determine how the crack’s movement is controlled by the atomic structure of the silicon.
As stress is applied at the preexisting crack, movement of the crack takes place through breaking of the adjacent interatomic bonds. Bernstein says, “The path of the crack is dependent upon the direction it moves through the silicon.”
Experimental and computer simulation focused initially on evaluation of the (111) crystal orientation. In the former, a silicon crystal was loaded into an aluminum frame and the (111) surface was fractured. Bernstein says, “We induced a crack that propagates through the silicon sample.”
Interestingly enough, triangular ridges are observed that all deviate in the same direction as the fractured surface at speeds below 800 meters per second. When the crack is propagated at higher speeds of 2,000 meters per second, no ridges are detected and the surface remains smooth.
In the computer simulations, the researchers found that the crack proceeded to move in a downward direction through the silicon atomic layers. Bernstein adds, “The crack propagates by moving down one plane at a time.” The reason for the crack moving down through the material is unknown at this point.
Prior to crack propagation, the silicon atoms form six-member rings with the same structure as diamond. Once the crack breaks the silicon bonds, the atoms near the crack tip will occasionally become reconstructed through the formation of five-member and seven-member rings if the crack is moving slowly. Bernstein says, “This is an energetically favorable way for the area disrupted by the crack to become reconstructed. It is similar to the formation of a dislocation.” An image of the reconstruction developed from the computer simulation is shown in Figure 1.
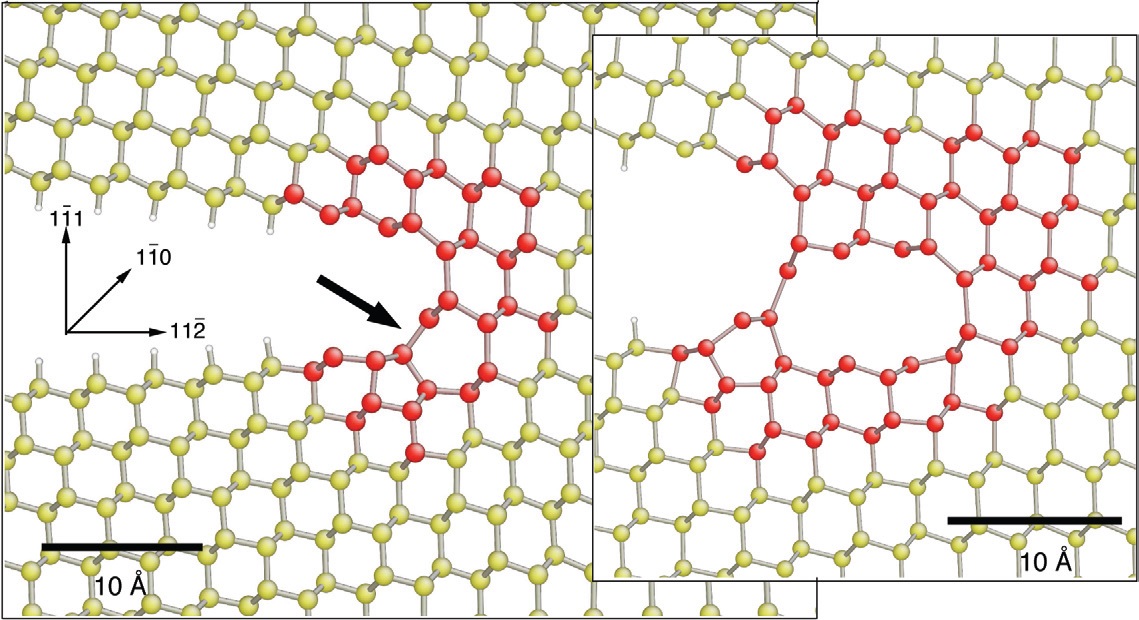 Figure 1. Crack propagation through a brittle silicon material containing six-member rings leads to a reconstruction process that generates five-member and seven-member rings. The red and yellow silicon atoms are described through computer simulations. (Courtesy of Nature and the Naval Research Laboratory)
Figure 1. Crack propagation through a brittle silicon material containing six-member rings leads to a reconstruction process that generates five-member and seven-member rings. The red and yellow silicon atoms are described through computer simulations. (Courtesy of Nature and the Naval Research Laboratory)
Bernstein noted that the reconstruction blunts the crack. This will reduce the driving force of the crack.
The computer simulations indicate that crack propagation should lead to the creation of macroscopic ridges visible in the silicon. This finding enables the researchers to explain the data generated from the experimental work.
Computer simulations show that the strength of the silicon bonds affects how the crack propagates through the material. In orientations where the stress is distributed on several bonds near the crack tip, the crack will go in a straight line only at low speeds when the weakest bonds always break first. At higher speeds other bonds may break, and the crack will diverge from a straight path. For other orientations, where the stress is concentrated only on bonds directly ahead of the tip, the crack moves in a straight line through the silicon.
This work shows that cracks growing at slow speeds will move in different orientations than at higher speeds. Such crack movements can affect the physical properties of the material on a macroscopic level.
The researchers are moving on to evaluate materials that are more relevant for use in materials such as silicon carbide, diamond, graphene and sapphire. Bernstein has been focusing on studying silicon carbide. He says, “We have been seeing similar reconstructions of the crack tip with silicon carbide as was observed with silicon. One of the most interesting aspects of working with silicon carbide is that the chemistry of the material is a more important factor because the two surfaces exposed by the crack are chemically inequivalent.” One surface is terminated with silicon atoms while the other surface ends with carbon atoms.
Most metals are ductile and exhibit atomic dislocations when stressed, according to Bernstein. He says, “We have found that even brittle materials can behave in a manner reminiscent of metals when dealing with cracks at low speeds.”
Further details on this work can be found in a recent paper (
1).
REFERENCES
1.
Kermode, J., Albaret, T., Sherman, D., Bernstein, N., Gumbsch, P., Payne, M., Csanyi, G., and De Vita, A. (2008), “Low-Speed Fracture Instabilities in a Brittle Crystal,”
Nature,
455 (7217), pp. 1224–1227.
 Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat items can be sent to him at neilcanter@comcast.net.