transition pressure and ii) a plateau value of the apparent viscosity for mean contact pressures higher than the glass transition pressure.
To contrast with this result, at the same pressure order of magnitude, the plateau regime cannot be experimentally captured for some lubricating fluid, as for the squalane on Figure 1.
This study aims at understanding and experimentally validating the mechanisms behind the onset of the friction plateau regime at high contact pressures.
METHODS: Tribological measurements are performed in a contact lubricated with squalane on a ball on disc tribometer. Experiments are designed, as for the benzyl benzoate4, in order to minimize thermal effects. These experiments are then analyzed in light of the squalane rheological characterization5.
RESULTS: Glass transition has been shown to be correctly predicted by the WLF Yasutomi model fitted on experimental data, for two fluids of different nature (the benzyl benzoate and a commercial turbine mineral oil supplied by Shell2,3). Indeed, the temperature dependent glass transition pressure Pg(T), derived from both the rheological model based on the 1012 Pa.s viscosity criterion and Brillouin spectroscopic measurements, led to the same orders of magnitude. Regarding the squalane rheological behavior, a WLF Yasutomi model previously provided in literature5 leads, for the same temperatures, to a Pg(T) always higher than 1.5 GPa. These predictions are correlated to friction curves to validate the strong link between the lubricant physical state and its macroscopic response.
DISCUSSION: To better understand the different rheological responses of these two fluids, their fragility 6 are studied versus both pressure and temperature and compared to strong and fragile reference fluids from literature. The benzyl benzoate shows a fragile behavior relatively to both parameters, whereas the squalane exhibits a strong behavior versus pressure. This lower pressure sensitivity could be appreciated in light of the fluid molecular architecture (see Figure 3).
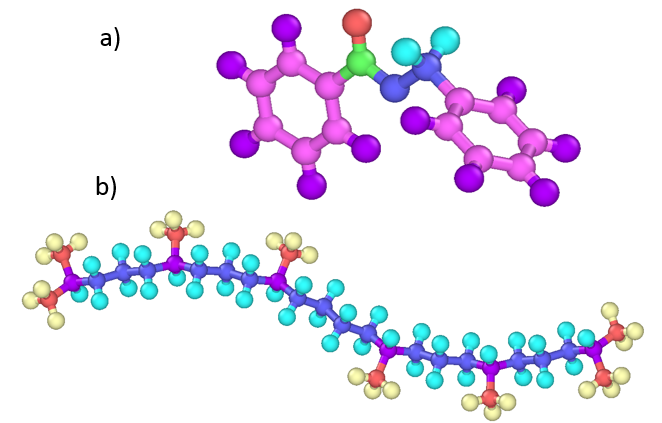
Figure 3 – Elementary molecules of the lubricants chosen in this study a) benzyl benzoate (ester of benzyl alcohol and benzoic acid) and b) squalane.
Indeed, the glass transition is described by Ediger7 as “a kinetic event which depends upon […] the time scales for molecular rearrangements”. Increasing pressure –thus density- in a sample would reduce the molecular mobility and consequently increase these rearrangement time scales. However, molecule architectures displayed on Figure 3 could explain the lower pressure sensitivity of this process for a linear hydrocarbon than for an ester with two benzene rings.
REFERENCES: 1. Martinie, Tribol. Lett. (2015), 2. NDiaye, to be submitted, 3. NDiaye, PhD Thesis (2017), 4. NDiaye, Tribol. Lett. (2017), 5. Bair, Tribol. Trans. (2017), 6. Bair, Proc. IMechE. (2007) 7. Ediger, J. Phys. Chem. (1996)