RESULTS: The data in Figure 1a presents the measured coefficient of friction for the self-mated PAAm hydrogels. The friction of the glass-molded hydrogel was always higher than the friction of the PDMS-molded gel and increased substantially with increasing sliding speed. On the other hand, the friction of the PDMS-molded gel remained close to the super-lubricity limit of 0.01 irrespective of the sliding speed. Nano-indentation experiments showed substantial differences in elastic moduli for the both hydrogel surfaces. The Young’s modulus of the glass-molded surface was about 11 kPa and remained relatively constant within 2 mm of the surface, whereas the PDMS-molded hydrogel was much softer and showed more of a gradient in modulus, with an average value of 0.3 kPa over the first few mm of depth. The ATR-IR results showed higher intensity of the amide peaks for the stiffer, glass-molded sample compared to the PDMS-molded one. Increasing the contact pressure from 0.1 to 10 kPa increased the signal intensity for both samples, however, the polymer-density seemed to remain lower for the PDMS-molded gel over the entire range of tested pressures. A similar result was also obtained with the NR, where the polymer density at the surface of the glass-molded sample remained higher than the density for the PDMS-molded sample at both tested contact pressures (0.1 and 10 kPa), Fig 1b.
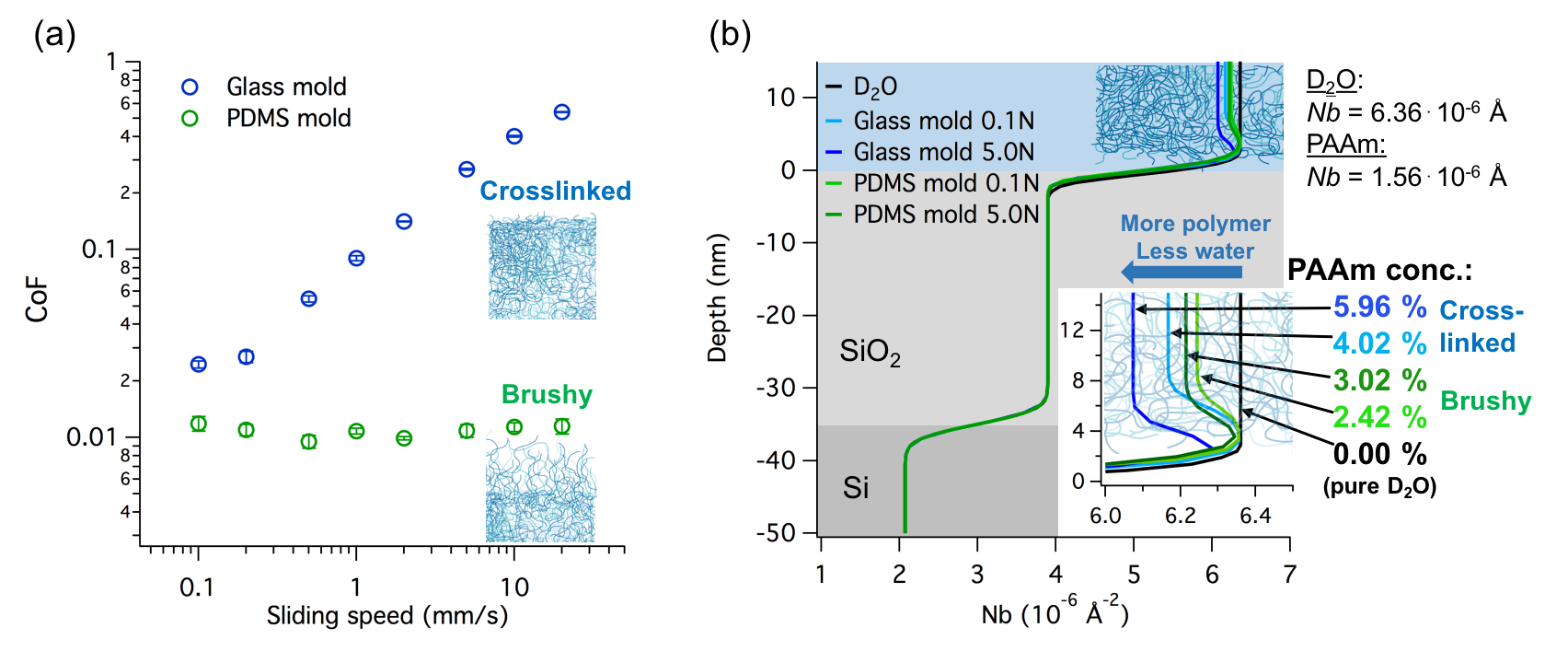
Figure 1 a) Coefficient of friction as a function of sliding speed for a glass-molded gel and a PDMS-molded gel in self-mated contact at » 6 kPa of contact pressure. b) Scattering-length density profile for the two gels obtained by fitting the neutron reflectivity curves of the two gel surfaces pressed against a silicon block at 0.1 kPa (0.1 N) and 10 kPa (5 N) pressure in water.
DISCUSSION: By polymerizing the PAAm hydrogels against a hydrophilic or a hydrophobic mold, we were able to affect the surface properties of otherwise similar bulk hydrogels. The much lower friction in the case of the gel from the hydrophobic mold implies a softer, brushier surface than in the case of the gel from a hydrophilic mold. This was confirmed by the much lower elastic moduli of the hydrophobically molded gel. Both ATR-IR and NR confirmed the lower polymer concentration in the case of the softer-surface gel. Furthermore, the IR and the NR results showed that the softer surface does not collapse easily under a contact pressure up to 10 kPa. This indicates that despite the softer structure, the surfaces of hydrophobically molded gels are able to resist exudation at low pressures, which is presumably responsible for the good lubricity of these kinds of gels.
REFERENCES:
1. J.P. Gong, Soft matter (2006) 544-552.
2. A.C. Dunn, Tribol Lett (2013) 371–378.
3. S. Lee, Science (2008) 575-576.
4. J.P. Gong, J. Phys. Chem. (2001) 4572-4576.
5. G. Sudre, Langmuir (2012) 12282-12287.
6. G.A. Ateshian, J Biomech. (2009) 1163-1176.
7. A.J. Shaw, Invest. Ophthalmol. Vis. Sci. (2010) 1911–1917.