INTRODUCTION:
Polyetheretherketone (PEEK) have been replacing many metallic components in industrial equipment such as seal rings, transmission gears, bushings, sliders, bearings and valves. Its good mechanical properties, light weight, chemical and heat resistance, low friction, and wear behaviour make it an ideal material for such applications1. It is well known that PEEK, PBI (polybenzoimidazole) polymer blends and PEEK+CF (carbon fibre) composites show significantly less wear than individual polymers. The wear mechanisms of these blend are however, unclear2,3. The structure and function of the transfer film formed on metal counterface from the blend, which is the key process for reducing friction and wear, have not yet been understood well. This study is aimed to understand the micromechanisms of transfer film formation of PEEK on steel conterface. Further studies are planned to elucidate transfer film formation of its blends.
METHODS:
A 6 mm diameter steel ball were slid against a polymer disc on a unidirectional pin-on-disc tribometer. Load, speed and temperature were varied to study their dependence on transfer evolution in a period of 30 minutes. Measurements of ball and disc temperature, charge and plasma luminescence were recorded using the setup shown in Fig 1. The ball material AISI52100 steel.
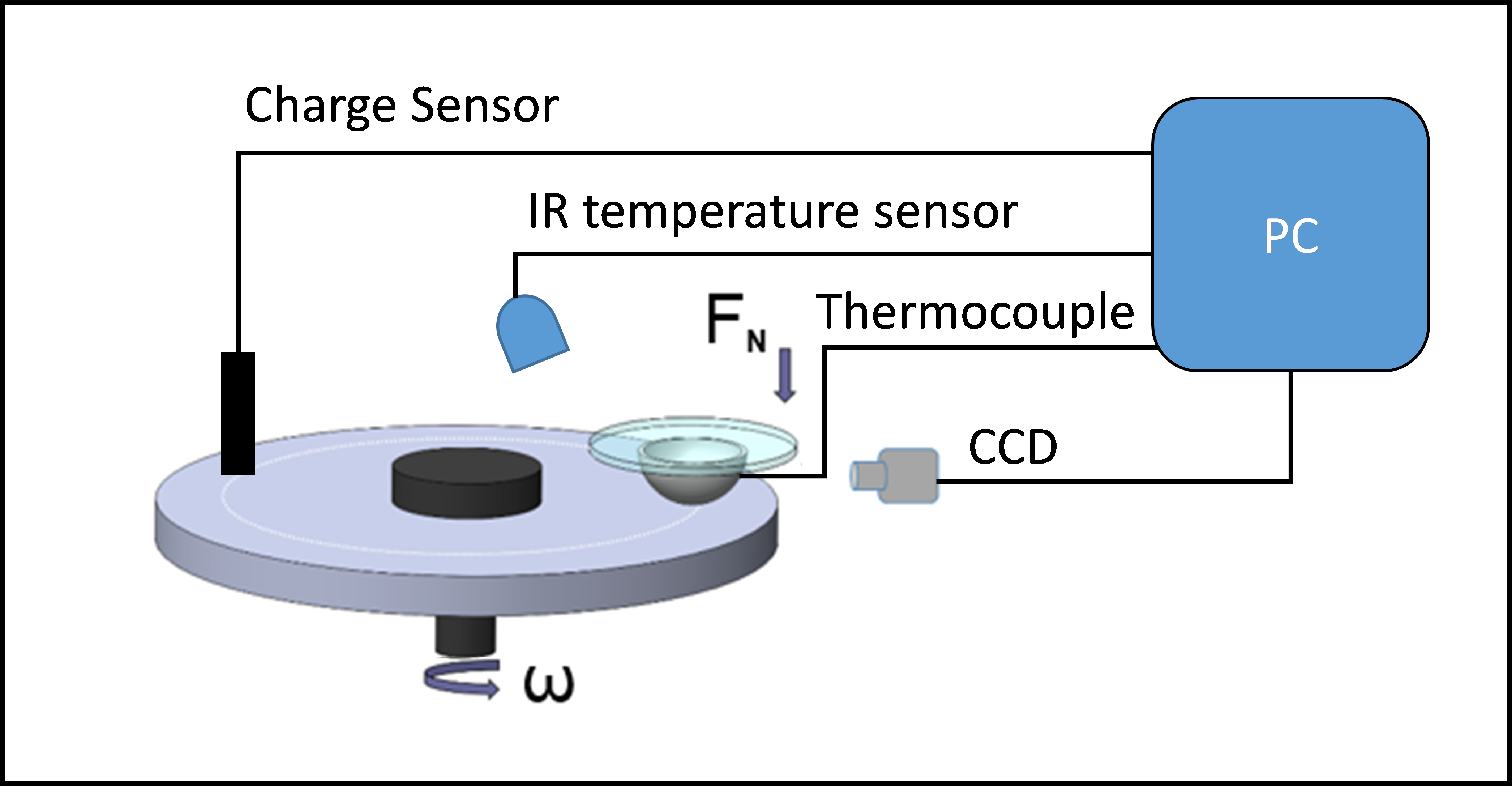
Figure 1: Schematic of Experimental set-up
RESULTS AND DISCUSSION:
When discs made of PEEK, PBI and their blends are rubbed against steel balls, time varying luminescence was observed at the contact interface. In samples containing CF, luminescence was completely absent which is attributed to the increase in conductivity of disc samples. The occurrence of luminescence suggests the generation of plasma in-situ termed as triboplasma4. Triboplasma is generated at the inlet as well as the outlet of the contact and is more intense at the contact outlet (see Figure 2).
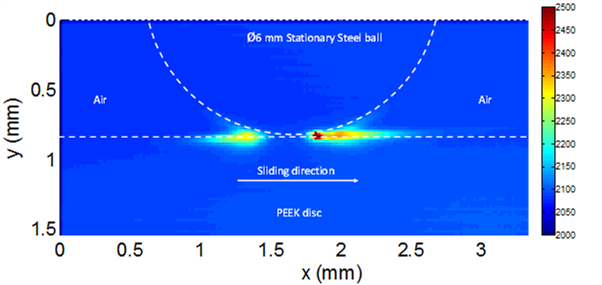
Figure 2 Distribution of average triboplasma emission around PEEK/Steel contact during sliding for 30 minutes at 6 N and 2 m/s, as observed from the side.