Direct borohydride fuel cells (DBFCs) have potential for use, but the key components (sodium borohydride and hydrogen peroxide) are not stable under the same pH conditions.
Implementation of a pH-gradient-enabled-microscale bipolar interface in the DBFC enabled both components to operate under optimum reaction conditions.
The operating voltage of a DBFC configured with this interface was double that of a typical proton exchange membrane (PEM) fuel cell.
In the effort to develop fuel cells as an effective alternative power source, research is ongoing to improve their performance. A good deal of attention has been spent on optimizing proton exchange membrane-based fuel cells, but other types also are under evaluation.
In a previous TLT article (
1), the challenge in improving the effectiveness of a solid oxide fuel cell involves improving the lanthanide, strontium, cobalt and iron cathode that is widely used to reduce oxygen. The efficacy of the cathode is hindered due to strontium segregation migration. Researchers improved the performance by applying a multiphase coating on the cathode, which reduced strontium migration and improved cathode performance and durability.
Direct borohydride fuel cells (DBFCs) are another appealing option because they exhibit high power density and operating voltages compared to other types. Vijay Ramani, the Roma B. and Raymond H. Wittcoff Distinguished University professor in the energy, environmental and chemical engineering department at Washington University in St. Louis, says, “DBFCs exhibit a theoretical voltage of 1.4 volts that is double the voltage (0.7 volt) of a typical hydrogen-based fuel cell. This means that in a typical application, only half the number of DBFC fuel cells need to be stacked, which can lead to a reduction in cost, weight and volume.” The latter three parameters are all very important in applications such as automobiles.
A DBFC relies on the use of sodium borohydride as the fuel and hydrogen peroxide as the oxidant. Sodium borohydride in many ways is a more convenient fuel than hydrogen gas. The challenge facing researchers is that sodium borohydride and hydrogen peroxide are stable under different pH conditions. Ramani says, “Sodium borohydride is stable at high pH but decomposes under neutral and acidic conditions. In contrast, hydrogen peroxide is not very stable under high pH conditions but is more stable under acidic conditions.”
The need for having a uniform pH environment has restricted the performance of most fuel cells because one of the two half reactions does not proceed at a rapid rate. For example, in using a proton exchange membrane fuel cell, the oxygen reduction reaction does not occur rapidly.
A second challenge in using DBFCs is the transport of sodium ions while using a cation exchange membrane separator. Ramani says, “Sodium is a big ion that is difficult to move through a fuel cell leading to greater resistance and reducing performance.”
To effectively have each half-reaction in a DBFC perform at an optimum level, the researchers have devised a new interface that enables both half-reactions to operate under ideal pH conditions.
pH-gradient-enabled-microscale bipolar interface
Ramani and his colleagues developed a DBFC that utilizes a pH-gradient-enabled microscale bipolar interface (
PMBI, see Figure 1), which enabled the sodium borohydride half reaction to occur at high pH and the hydrogen peroxide half reaction to take place at low pH. In the PMBI configuration, a cation exchange separator is placed in close contact with an anion-exchange-binder-covered electrocatalyst.
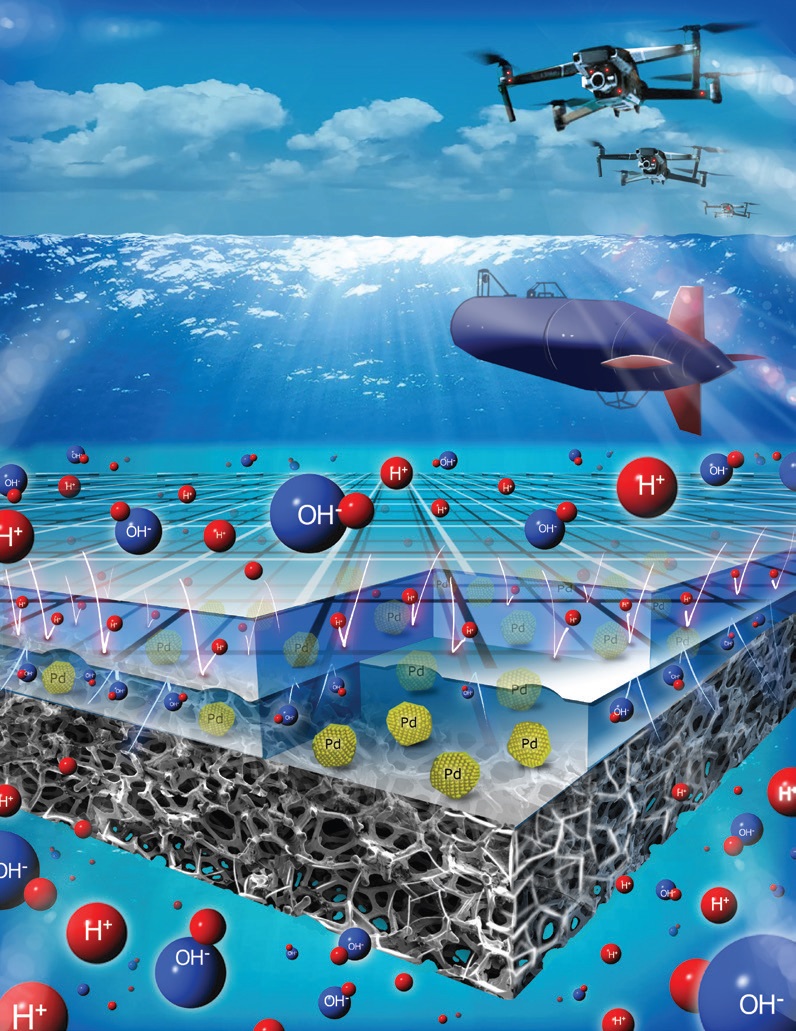 Figure 1. This artist’s representation of a pH-gradient-enabled microscale bipolar interface was used to improve the performance of a direct borohydride fuel cell. (Figure courtesy of Washington University.)
Figure 1. This artist’s representation of a pH-gradient-enabled microscale bipolar interface was used to improve the performance of a direct borohydride fuel cell. (Figure courtesy of Washington University.)
The researchers used a palladium/carbon catalyst to facilitate the sodium borohydride reaction at the anode. The purpose of the anionic-exchange-binder is to block hydrogen cations from reaching the anode enabling the local pH to remain around 13.5.
With the PMBI established, there may be concern that ionic transport between the anode and the cathode is not permitted. Ramani says, “The junction potential at the PBMI enables water to be split into hydrogen and hydroxide ions that migrate to the cathode and anode sides, respectively enabling them to participate in both half-reactions.”
The thickness of the PMBI is important to ensure that the pH distribution is reasonable. Ramani says, “We prepared a PMBI with a thickness of 10 nanometers. Our objective was to produce a uniform pH distribution at the PMBI. Transmission electron microscopy showed that the pH gradient distribution ranged from 0.5-3.3 pH units with the average gradient being 0.82 pH units per nanometer.”
The researchers evaluated the PMBI-based DBFC versus configurations run entirely at low pH and at high pH. In evaluation testing, the PMBI-configured fuel cell exhibited superior performance with an open circuit voltage of 1.8 volts and an estimated power density of about 500 milliwatts per square centimeter at 1.5 volts (double the operating voltage of a typical proton exchange membrane [PEM] fuel cell).
Both the low pH and high pH DBFCs displayed inferior performance due to problems in the ability of both cells to maintain the desired pH conditions. Ramani says, “We have evaluated the PMBI DBFC at the lab scale for up to 100 hours and found the cell to perform well with no decline in performance.”
The researchers optimized the performance of the DBFC through changing the thickness of the separation membrane and by optimizing the flow rates. Ramani says, “By increasing the flow rate, we were able to more effectively distribute the reactants among the active catalyst sites.”
The researchers will be working to make the PMBI thickness distribution more homogeneous and also develop newer cell designs to improve performance and scale up the DBMF for use in industrial applications. Additional information can be found in a recent article (
2) or by contacting Ramani at
ramani@wustl.edu.
REFERENCES
1.
Canter, N. (2018), “Enhancing the Rate of Oxygen Reduction in Fuel Cells,” TLT,
74 (6), pp. 22-23.
2.
Wang, Z., Parrondo, J., He, C., Sankarasubramanian, S. and Ramani, V. (2019), “Efficient pH-gradient-enabled Microscale Bipolar Interfaces in Direct Borohyride Fuel Cells,”
Nature Energy, 4, pp. 281-289.