Higher-performing lithium-ion batteries
Dr. Neil Canter, Contributing Editor | TLT Tech Beat March 2018
Use of a lithium-rich cation oxide as a cathodic material may significantly increase the battery's energy density.
 © Can Stock Photo / dmitrimaruta
© Can Stock Photo / dmitrimaruta
KEY CONCEPTS
•
The cathode of the lithium-ion battery must be upgraded to improve performance.
•
A new material based on lithium, iron and oxygen has been developed that can theoretically double the energy density of the lithium-ion battery.
•
The ability of this material to enable lithium ions to move reversibly was predicted by computational studies and confirmed by experimentation.
In the development of lithium-ion batteries, researchers are not only looking for safer approaches but also to boost battery performance. Battery safety has emerged as a key concern, and this column has discussed a number of solutions.
The main safety issue with lithium-ion batteries is the flammability and toxicity of the organic liquid electrolyte. In a previous TLT article, researchers developed a lithium-ion battery with an aqueous electrolyte (
1). To minimize the evolution of hydrogen gas at the anode through water decomposition, the researchers coated the anode with a strongly hydrophobic gel that strongly repelled water.
To improve lithium-ion battery performance, the focus must be on the cathode. Christopher Wolverton, Jerome B. Cohen professor of material science and engineering at Northwestern University in Evanston, Ill., says, “The cathode enables the highest energy density of lithium-ion batteries. A typical lithium-ion battery generates a four-volt reaction using lithium cobalt oxide as the main cathode.”
Wolverton points out that lithium cobalt oxide has been used for more than 25 years, and researchers have been trying to replace it ever since it was developed. He says, “The high cost and toxicity of cobalt have been two reasons for finding a replacement. Another issue is that the maximum amount of lithium cannot be used reversibly when utilizing this cathode material. While the stoichiometry shows that there is one lithium atom per cobalt atom, in actuality the crystal structure for lithium cobalt oxide falls apart when all of the lithium is removed. In today’s lithium-ion batteries, only 0.5 lithium atom is removed leaving 0.5 lithium atom sitting there in the molecule and not doing anything.”
An attractive alternative is to replace the cobalt atom with iron. Additionally, getting oxygen to participate in the reaction offers new opportunities. Wolverton says, “If we can find compounds where oxygen can participate in the reaction storing and releasing electrical energy as lithium ions move back and forth between the cathode and the anode, then there is the potential for using more lithium and producing a higher capacity battery. We chose iron as the transition metal because it is cheaper than cobalt.”
Past attempts to use oxygen in this manner in iron-containing compounds have failed because the resulting compounds would become unstable and the reactions involving oxygen were irreversible.
One option that has been evaluated is a material with five lithium atoms, four oxygen atoms and an iron atom (Li
5FeO
4). Wolverton says, “This compound has a completely defined crystal structure that, when lithium is partly removed, converts to a disordered rock salt. The appeal of this compound is the presence of five lithium atoms per iron instead of the 1:1 ration of lithium to cobalt that is currently used. When Li
5FeO
4 is used as a traditional cathode, oxygen is lost as well in an irreversible manner, meaning that this salt cannot be used in a lithium-ion battery.
Further work has now shown that conversion of Li
5FeO
4 to another salt with a different stoichiometry produces a salt that will work and may lead to a higher performing lithium-ion battery.
Oxygen not released
Wolverton and his research team, in collaboration with researchers at Argonne National Laboratory, determined that a derivative of Li
5FeO
4 is a feasible option for use as a cathodic material in lithium-ion batteries. He says, “Our Argonne collaborators spent a good deal of time evaluating salts that can reversibly move oxygen in and out of the material through experimentation. They found that removing a small amount of oxygen from Li
5FeO
4 leads to the formation of a new material that will enable lithium ions to move reversibly but not release oxygen.”
In effect, 0.5 equivalent of lithium oxide was removed from Li
5FeO
4 to produce the desired compound, which has the chemical structure, Li
4FeO
3.5. Wolverton says, “This is not a naturally occurring compound but forms during the process of activation of Li
5FeO
4.”
The researchers found that the salt with four lithium atoms undergoes a reversible redox reaction as shown in Figure 1. One lithium atom undergoes the exchange, which theoretically leads to the possibility of using twice the energy density in operating the battery as compared to the currently used, lithium cobalt oxide.
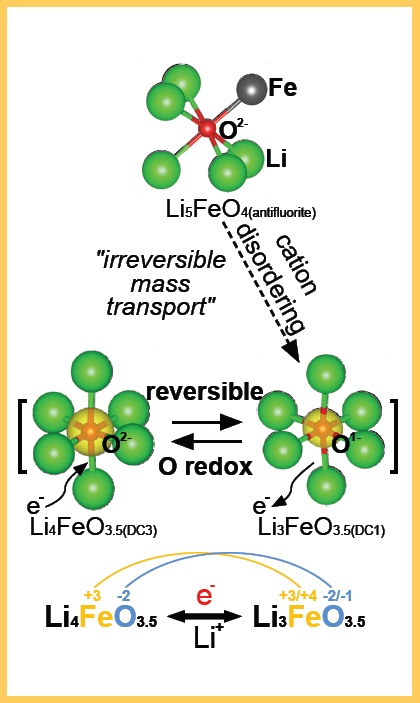 Figure 1. Activation of Li5FeO4 through an irreversible process leads to the formation of a potential cathodic material, Li4FeO3.5, that can enable lithium ions to move reversibly without releasing oxygen. (Figure courtesy of Northwestern University.)
Figure 1. Activation of Li5FeO4 through an irreversible process leads to the formation of a potential cathodic material, Li4FeO3.5, that can enable lithium ions to move reversibly without releasing oxygen. (Figure courtesy of Northwestern University.)
Wolverton and his colleagues conducted computational studies to predict the reversibility of this reaction, which were confirmed by the experiments from Argonne. The presence of four lithium atoms in the salt makes it theoretically possible that a larger number of lithium ions could be involved, further increasing the battery’s energy density. Wolverton says, “This objective cannot currently be achieved with Li
4FeO
3.5 empirically. We can only get the one lithium atom to interact reversibly.”
Future work will involve evaluating other lithium-rich cation oxides that can undergo redox without releasing oxygen. Wolverton says, “Through our use of computational analysis, we can screen a large number of materials in a short period of time.”
Additional information on this research can be found in a recent article (
2) or by contacting Wolverton at
c-wolverton@northwestern.edu.
REFERENCES
1.
Canter, N. (2018), “Safer lithium-ion batteries,” TLT,
74 (1), pp. 14-15.
2.
Zhan, C., Yao, Z., Lu, J., Ma, L., Maroni, V., Li, L., Lee, E., Alp, E., Wu, T., Wen, J., Ren, Y., Johnson, C., Thackeray, M., Chan, M., Wolverton, C. and Amine, K. (2017), “Enabling the high capacity of lithium-rich anti-fluorite lithium iron oxide by simultaneous anionic and cationic redox,”
Nature Energy,
2 (12), pp. 963-971.
Neil Canter heads his own consulting company, Chemical Solutions, in Willow Grove, Pa. Ideas for Tech Beat can be submitted to him at neilcanter@comcast.net.