friction modifier. The ECR suggests that the organic friction modifier forms a boundary lubricating film which effectively separates the two opposing surfaces. On the other hand, little or no surface separation is achieved in the base oil alone.
Upon heating there is a sudden transition at 118 °C in the coefficient of friction of the base oil– the large fluctuations cease and the value drops to 0.176. With continued time spent at elevated temperature, the coefficient of friction continues to fall, before stabilizing upon cooling to 100 °C at a value of 0.129. During the test, the ECR increases, suggesting that a boundary lubricating film forms.
Transitions are also seen during the test with friction modifier, however they are less obvious in the graph plotted on this scale. With heating there is a gradual decrease in the coefficient of friction. A sharp decrease in friction occurs at a temperature of 125 °C, which is accompanied by a drop in the ECR, suggesting either changes in or disruption of the organic friction modifier boundary lubricating film. The coefficient of friction reaches a minimum value of 0.05 at a temperature of 150 °C, before subsequently increasing with sliding time. After the heating-cooling cycle, the coefficient of friction (0.110) is higher than its original value. Large fluctuations in the ECR hint at instability within the boundary lubricating film.
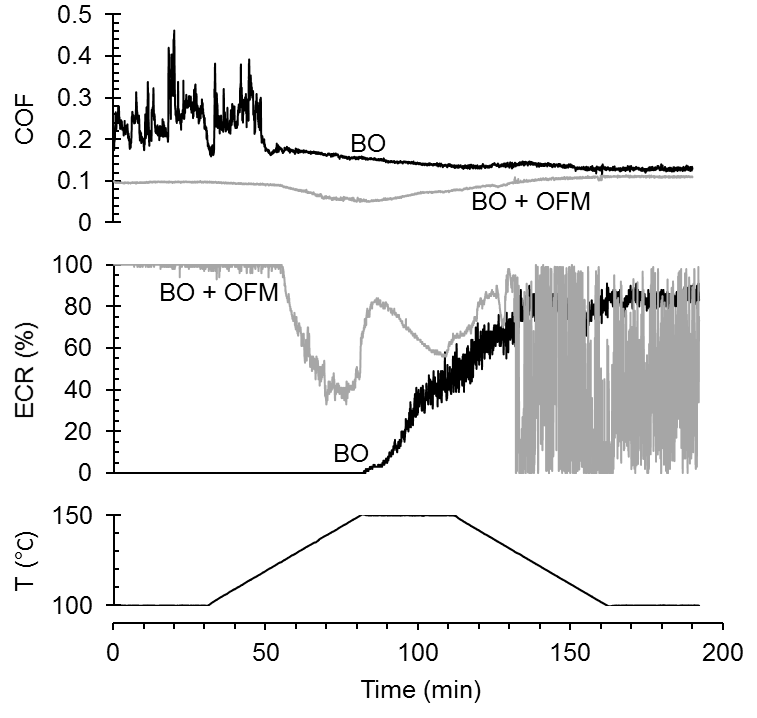
Figure 1 – High temperature HFRR friction experiments in base oil (BO) and base oil with organic friction modifier (BO + OFM). Graphs show coefficient of friction (COF, top), electrical contact resistance (ECR, middle) and temperature (T, bottom) vs. time.
DISCUSSION: These experiments support that some degree of oxidation of unadditivated base oil is favorable since it facilitates boundary lubricating film formation. However, oxidation species can have a negative impact the performance of organic friction modifier additive. Further studies investigate base oil oxidation by spectroscopic techniques.
REFERENCES: 1. Björling, Proc. IMechE Part J 231, 708 (2017), 2. Lahijani, ASLE Trans. 25, 25 (1982), 3. Blaine, Ind. Eng. Chem. Res. 30, 2185 (1991), 4. Chen, Tribol. Lett. 14, 83 (2005), 5. Pfaendtner, Ind. Eng. Chem. Res. 47, 2886 (2008), 6. Diaby, Carbon 47, 355 (2009), 7. Hsu, Tribol. Int. 38, 305 (2005) 8. Walling, Acc. Chem. Res. 8, 125 (1975). 9. Vinogradov, J. Basic Eng. 87, 741 (1965), 10. Hsu, Chapter 12 of Modern Tribology Handbook, CRC Press (2001), 11. Spikes, Lubrication Sci. 2, 3, (1989).