The depth and morphology of the wear tracks on the disc specimens were investigated by optical profilometry. The elemental distribution across the wear track was investigated using an Auger electron spectroscope with a 10 keV electron beam.
RESULTS
The amount of hydrogen (in ppm) present in the disc specimens after the 10 hour RCF tests is shown in Figure 2. Results show that the concentration of hydrogen in the material is related to the affinity of the base oil to the wear track, and its susceptibility to degenerate in the contact and produce atomic hydrogen. Tests performed in hydrogen gas show a larger variation of hydrogen in the material as compared to tests conducted in air, indicating that the environmental gas is an important factor.
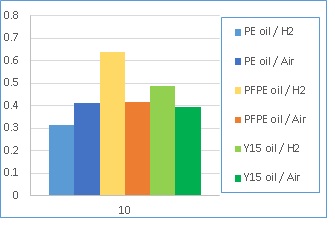
Figure 2 - Hydrogen content (ppm) in the disc specimens after the RCF tests (10 h) measured by Thermal desorption spectroscopy.
Base oil 1 is a polyester and is attracted to the surface of the metal easily. This provides competition to any chemical reactions that would allow the generation of atomic hydrogen. As a result, the concentration of hydrogen in the material does not increase substantially during operation.
Base oils 2 and 3 are fluorinated oils and their affinity towards the interface is reduced. However, the decomposition of these molecules creates various chemical species that can corrode the interface and thus influence hydrogen generation. In this case the amount of hydrogen measured in the samples after the tests varies substantially.
The morphology and chemical composition on the wear tracks was also investigated in order to determine the chemical reactions that take place in the tribological contact in each case.
Understanding the behaviour of steel components in hydrogen environments and designing technologies capable of improving their performance and life cycle constitutes the next important challenge towards a clean and efficient hydrogen based economy in the future.
REFERENCES
1. Tanaka, Niste, Abe, Sugimura, Tribol. Lett., 65, 94, (2017),
2. Niste, Tanaka, Ratoi, Sugimura, RSC Adv., 5 (51), 40678-40687, (2015),
3. Tanaka, Morofuji, Enami, Hashimoto, Sugimura, Tribol. Online, 8(1), 90–96, (2013).