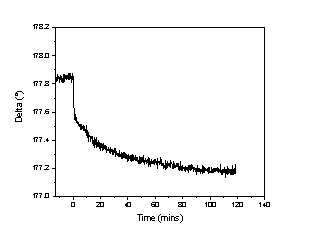
Figure 2 – The change in Delta Δ after the addition of 1 mM octadecylamine in hexadecane at t = 0 to a solution of hexadecane on silicon.
RESULTS & DISCUSSION: A baseline is taken in pure hexadecane and once the octadecyalmine solution is added (t = 0), Δ drops, as shown in Figure 2. The decrease in delta with minimal change in Ψ shows a growth of a thin film on the surface8. The surface can then be modeled presuming a homogenous layer is growing on the surface to give a thickness to the layer grown. The final height of the SAM is estimated to be 1.0 - 1.25 nm, suggesting a tilt angle of the molecules to be 60 - 67° from normal.
This results from 1 mM is in agreement with previous AFM results of octadecylamine on mica 10 which shows the formation of islands with a height of 0.9 to 1.4 nm.
This shows that Ellipsometry can be used for measuring the growth of the monolayer from hexadecane solutions despite the refractive index values being so close.
REFERENCES: [1] S. M. Lundgren, K. Persson, B. T. Kronberg, and P. M. Claesson, Tribol. Lett., vol. 22, no. 1, pp. 15–20, 2006. [2] M. H. Wood, M. T. Casford, R. Steitz, A. Zarbakhsh, R. J. L. Welbourn, and S. M. Clarke, Langmuir, vol. 32, no. 2, pp. 534–540, 2016. [3] H. Brunner, T. Vallant, U. Mayer, and H. Hoffmann, J. Colloid Interface Sci., vol. 212, no. 2, pp. 545–552, 1999. [4] V. A. Gilchrist, J. R. Lu, J. L. Keddie, E. Staples, and P. Garrett, Langmuir, vol. 16, no. 2, pp. 740–748, 2000. [5] D. Li, X. Song, J. Xu, Z. Wang, R. Zhang, P. Zhou, H. Zhang, R. Huang, S. Wang, Y. Zheng, D. W. Zhang, and L. Chen, Appl. Surf. Sci., vol. 421, pp. 884–890, 2017. [6] M. L. Miranda-Medina, S. Spiller, A. Vernes, and M. Jech, Tribol. Int., vol. 113, no. July 2016, pp. 101–110, 2017. [7] S. R. Wasserman, G. M. Whitesides, I. M. Tidswel1, B. M. Ocko, P. S. Pershan, and J. D. Axel, J . Am. Chem. SOC, vol. 1, no. 1, pp. 5852–5861, 1989. [8] H. G. Tompkins, Handbook of Ellipsometry, vol. 30, no. 7. Springer Berlin Heidelberg, 2005. [9] L. Asinovski, D. Beaglehole, and M. T. Clarkson, Phys. Status Solidi Appl. Mater. Sci., vol. 205, no. 4, pp. 764–771, 2008. [10] S. Campen, J. H. Green, G. D. Lamb, and H. A. Spikes, Tribol. Lett., vol. 58, no. 3, 2015.